Use of the Aβ1-42/Aβ1-40 ratio to improve accuracy of AD diagnosis
It is widely agreed that, since cerebrospinal fluid (CSF) is in direct contact with the central nervous system (CNS), analytes measured in this body fluid can be considered as a good biomarker for a disease like Alzheimer’s disease (AD), if well analysed and accepted parameters are used. Biomarkers that come closest to fulfilling these criteria are amyloid β peptides (Aβ peptides) and Tau proteins. This can easily be explained by the involvement of these proteins in pathologic events directly associated with the disease, namely deposition of senile plaques and formation of neurofibrillary tangles. Involvement of these proteins in disease development is known and has been investigated intensively for many years. However, there is no single marker that can differentiate AD from other neurodegenerative disorders or predict the risk of progression to AD. In this article series we would like to summarize some facts about the use of CSF amyloid β peptides ending at positions 42 and 40 (Aβ1-42 and Aβ1-40, respectively) in combination as Aβ1-42/Aβ1-40 ratio and demonstrate that:
- it is significantly better than CSF Aβ1-42 alone to detect brain amyloid deposition in AD
- it can help in differentiating AD dementia from non-AD dementias
- it reduces variability due to preanalytical differences.
Read the first post below or download the complete guidance document now!
Follow our articles on this topic over the next weeks and get deeper information on the value of using Aβ1-42/Aβ1-40 ratio.
Or simply download the complete guidance document now! (requires a Premium eServices account)
CSF Aβ1-42/Aβ1-40 ratio as a tool for detecting amyloid deposit
- The distribution of total Aβ (40 and 42) follows a Gaussian distribution for both normal subjects and AD patients.
- Use of the Aβ1-42/1-40 ratio can lead to more correctly diagnosed AD independently of high or low levels of total Aβ load in the patient.
In a population of normal subjects and AD patients, the distribution of total Aβ (40 and 42) follows a Gaussian distribution for both normal subjects and AD patients, with Aβ1-40 making up about 70% of total Aβ load.7 Although many cases fall within +/- 2SD of the Gaussian distribution with the majority having normal total Aβ, outliers are still present. Some AD patients will have high total Aβ and some will have low total Aβ. This is also true for the normal subjects.
This means that AD patients with a high total Aβ will be diagnosed as false negative and vice versa, normal subjects with low total Aβ will be diagnosed as false positive for AD, if only Aβ1-42 is used.
In all three cases (normal, low, and high total Aβ) the ratio can correctly diagnose for AD in more cases than Aβ1-42 alone.
CSF concentrations of Aβ1-42 and Aβ1-40, respectively, and total Tau (tTau) protein were measured by ELISA to compare their accuracy in discriminating patients with clinically diagnosed AD, non-AD, and control subjects. For all groups, the Aβ1-42/Aβ1-40 ratio classified more patients correctly, as compared to the concentration of Aβ1-42 alone: AD versus controls, 94 and 86.7%; AD versus non-AD, 90 and 85% and AD versus non-AD plus controls, 90.8 and 87%, respectively. The percentage of correctly classified patients was further improved when the Aβ ratio was combined with the analysis of tTau.3
Wiltfang et al. concluded that the amyloid β concentration ratio should replace the ‘raw’ concentrations of corresponding Aβ peptides to improve reliability of the neurochemical dementia diagnosis. A proof-of-principle study was carried out in 2007, which showed the correlation with tTau and pTau.8
More recently, several studies demonstrated added value for CSF Aβ1-40 or CSF Aβ1-42/Aβ1-40 ratio for differential diagnosis of AD using CSF pTau levels or in ambiguous AD CSF biomarker profiles.1
These and many other results show that the diagnostic accuracy of decision trees that contain the Aβ1-42/1-40 ratio are significantly better than the diagnostic accuracy of the decision tree without Aβ1-40 or the Aβ1-42/1-40 ratio. This was shown also in the study by Slaets et al. in 2013 comparing the use of different biomarkers for the diagnosis of AD. In this study, the CSF Aβ1-42/1-40 ratio has been added to the existing panel of biomarkers, Aβ1-42, tTau, and pTau and was compared to the panel without the ratio.7
Association between CSF Aβ1-42/Aβ1-40 ratio and amyloid PET
- A significant number of samples shows a mismatch between CSF Aβ1-42 and amyloid PET.
- Use of the Aβ1-42/Aβ1-40 ratio can increase the agreement both for people who are classified as positive (by low CSF Aβ1-42) and for people who are classified as negative (by high CSF Aβ1-42) but do not show the same classification in PET.
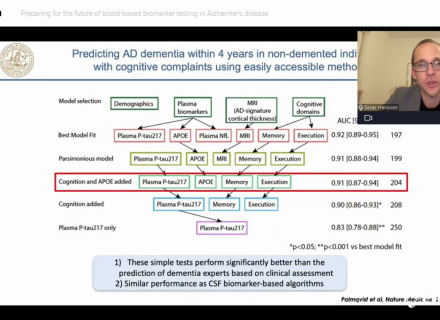
Most studies have found a strong association between CSF Aβ1-42 and amyloid PET measurements. However, in these studies, 10–20% of healthy individuals and MCI patients show a mismatch in CSF Aβ1-42 and amyloid PET status (Palmqvist et al. 2014).5 A study published by Janelidze et al. in 2016 could demonstrate that the CSF Aβ1-42/Aβ1-40 (and Aβ1-42/Aβ1-38) ratio better predict abnormal cortical amyloid deposition (visualized with PET) compared to Aβ1-42 alone. The ratios increased the classification performance both for people who were falsely classified as positive (by low CSF Aβ1-42) and for people who were falsely classified as negative (by high CSF Aβ1-42).
Janelidze et al. show that in patients with mild cognitive complaints, low CSF Aβ1-42, Aβ1-40, and Aβ38 are linked to subcortical injury. Underlying mechanisms are regarded to likely be related to dysregulation in APP pathways with a general decline in the production of Aβ. However, in accordance with earlier investigations, they found that low CSF levels of Aβ1-42, but not Aβ1-40 and Aβ38, were associated with more AD-specific neurodegeneration (i.e., hippocampal atrophy).
Pannee et al. demonstrated in 2016 that comparing CSF Aβ1-42 concentrations with 18F-flutemetamol PET showed high concordance with an area under the receiver operating characteristic curve of 0.85 and a sensitivity and specificity of 82% and 81%, respectively. The ratio of Aβ1-42/Aβ1-40 or Aβ1-42/Aβ1-38 significantly improved concordance with an area under the receiver operating characteristic curve of 0.95 and a sensitivity and specificity of 96% and 91%, respectively. These results show that the CSF Aβ1-42/Aβ1-40 and Aβ1-42/Aβ1-38ratios using the described MS method are strongly associated with cortical Aβ fibrils measured by 18F-flutemetamol PET.4 In addition, it has been shown that the Aβ1-42/Aβ1-40 ratio reflects an altered Ab kinetics and that this allows an AD diagnosis before PET amyloid deposition is detectable (Patterson et al. 2015).6
Altogether, these results indicate that an isolated drop in CSF Aβ1-42 is more specific for AD-type pathology, whereas lower CSF levels of all three Aβ isoforms might be associated with subcortical damage in general. This assumption fits their finding that reduced levels of all three Aβ species can be found in Parkinson Dementia/Dementia with Lewy bodies and Vascular Dementia, so in several disorders that are accompanied by subcortical changes.2
References
- Dorey, Aline, et al. "Association of cerebrospinal fluid prion protein levels and the distinction between Alzheimer disease and Creutzfeldt-Jakob disease." JAMA neurology 72.3 (2015): 267-275.
- Janelidze, S., et al. "for the Swedish BioFINDER, study group, Oskar, Hansson (2016) CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: Better diagnostic markers of Alzheimer disease." Ann Clin Transl Neurol 3: 154-165.
- Lewczuk, Piotr, et al. "Neurochemical diagnosis of Alzheimer’s dementia by CSF Aβ42, Aβ42/Aβ40 ratio and total tau." Neurobiology of aging 25.3 (2004): 273-281.
- Pannee, Josef, et al. "Reference measurement procedure for Csf amyloid beta (aβ) 1–42 and the Csf Aβ1–42/aβ1–40 ratio–a cross‐validation study against amyloid Pet." Journal of neurochemistry 139.4 (2016): 651-658.
- Janelidze, S., et al. "for the Swedish BioFINDER, study group, Oskar, Hansson (2016) CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: Better diagnostic markers of Alzheimer disease." Ann Clin Transl Neurol 3: 154-165.
- Patterson, Bruce W., et al. "Age and amyloid effects on human central nervous system amyloid‐beta kinetics." Annals of neurology 78.3 (2015): 439-453.
- Slaets, Sylvie, et al. "Cerebrospinal fluid Aβ 1-40 improves differential dementia diagnosis in patients with intermediate P-tau 181P levels." Journal of Alzheimer's Disease 36.4 (2013): 759-767.
- Wiltfang, Jens, et al. "Highly conserved and disease‐specific patterns of carboxyterminally truncated Aβ peptides 1–37/38/39 in addition to 1–40/42 in Alzheimer's disease and in patients with chronic neuroinflammation." Journal of neurochemistry 81.3 (2002): 481-496.