CSF biochemical pattern interpretation
In this article series we aim at highlighting the current state of knowledge and the latest developments in the field of Alzheimer’s disease (AD) testing. This chapter reviews some of the best-practices of CSF biochemical pattern interpretation.
Other articles in this series:
- Altered proteins in brain neurodegenerative diseases
- Using CSF biomarkers to link pathology and clinical presentation
- How to perform a lumbar puncture
- Handling and transportation of CSF samples
- Aβ deposition and clearance: a key feature of ageing brain
- New criteria for Alzheimer’s disease
Alzheimer’s disease and pre-clinical stages
Alzheimer’s disease is the most common neurodegenerative disease and demonstrates exponential increase in prevalence with advancing age beyond 60 years. There are three stages usually described: latent stage when the disease has started but is asymptomatic, prodromal stage when the disease has progressed and very mild clinical signs and symptoms are present, and the clinical stage when the disease has advanced and the full clinical spectrum is expressed.
It is critical to realize that latent disease cannot be distinguished from absence of disease by clinical examination or neuropsychological testing, but rather requires some ensemble of laboratory-based methods to detect disease initiation in the absence of symptoms.
How fast the patient will progress in the disease depends on risk-enhancing factors, such as age, modifying genes, cognitive reserve, comorbidities, and so forth.
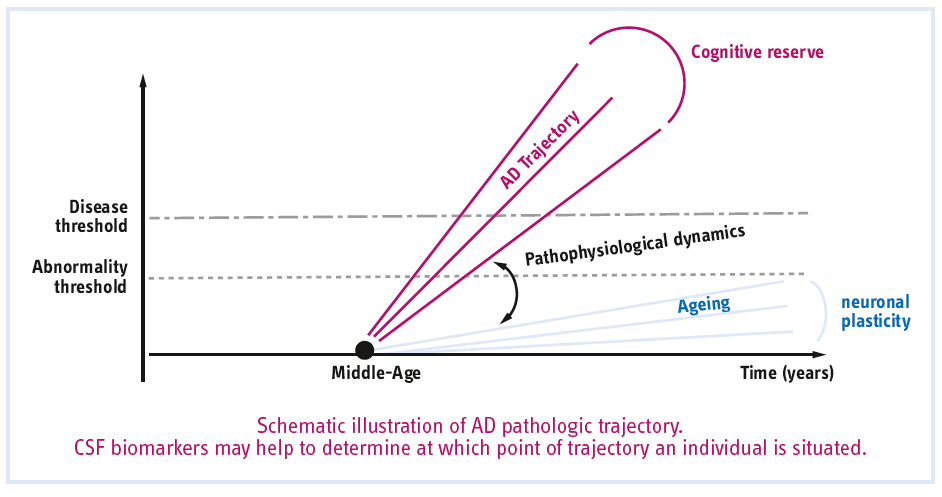
Pathophysiological biomarkers
Individuals can now be identified as being in the preclinical state by the in vivo evidence of Alzheimer pathology, by a biological or molecular “signature” of AD.
CSF Aβ42 and amyloid PET are highly concordant when used to dichotomize individuals as amyloid positive or amyloid-negative, showing 80%–90% agreement across studies. The CSF Aβ42/40 ratio typically shows agreement with PET above 90%.
A Tau PET ligand is not routinely available, CSF T-tau and P-tau are the easiest tools for evidence of tauopathy. Alternatively, topographical markers include volume changes in the brain (hippocampal atrophy, cortical thickness) assessed by MRI and hypometabolism of neocortical regions measured by fluorodeoxyglucose (FDG)-PET.
Optimal and reliable blood-based biomarkers are not yet ready for clinical application.
Only the association of both pathologic hallmarks defines AD even in the absence of cognitive symptoms.
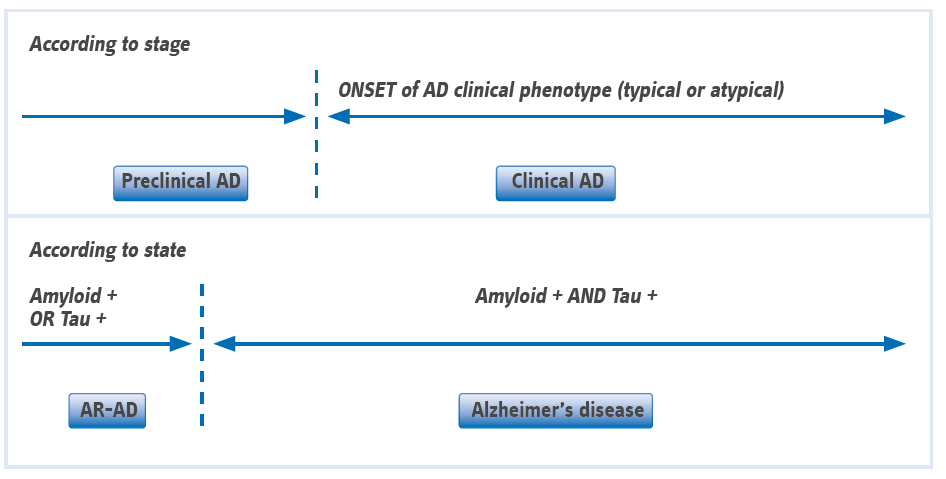
(From Dubois B, et al. Alzheimers Dement (2016) Abbreviations: AD, Alzheimer’s disease; AR-AD, at risk for AD)
Definition of abnormality threshold for CSF biomarkers
The threshold for “abnormality” for CSF biomarkers is difficult to assess, especially in clinically healthy elderly subjects. There are several approaches to define what is abnormal.
- abnormality may be defined based on comparison between cognitively normal (having a CSF collection for any other causes than NDD) and AD groups.
- abnormality can be defined based on the distribution of values within a cognitively normal population, where subjects with values exceeding, for example, 2 standard deviations below or above the mean can be considered “abnormal”.
- abnormality can be defined based on longitudinal observation of clinical progression in a group starting as healthy and declining to AD at follow-up evaluations.
In healthy control subjects, the cortical uptake of Aβ agents is low in comparison with patients suffering from prodromal AD of the hippocampal type/MCI-due-to-AD(*) or fully developed AD dementia. However, a significant proportion of cognitively healthy elderly show increased cortical Aβ binding and decreased CSF Aβ42. This finding is supported by postmortem histopathological data showing Aβ plaques upwards of 30% of the non-demented elderly population above 75 years of age, likely representing preclinical AD. (*) MCI-due-to-AD, Mild cognitive impairment due to Alzheimer etiology.
CSF T-tau levels increase with age and are higher in apolipoprotein E (APOE) carriers. The APOE polymorphism is the most widely accepted genetic factor increasing the risk for sporadic AD. APOE ε4 carriers might be predisposed to vascular diseases which in turn could contribute to age-related brain damage and therefore to elevated T-tau levels.
In conclusion, a substantial number of healthy subjects over age 60 (25-40%) has at least one CSF biomarker concentration in range that can be considered abnormal. To minimize the age-related risk factor, “normality” may be defined using results from clinically and cognitively normal individuals below the age of 50.
Remark: Commercial assays for the measurement of CSF biomarkers bearing the CE mark for in vitro diagnostics propose an estimated range of normal values for specific populations.
Combination of CSF biomarkers to be more useful in prediction
CSF T-tau, P-tau, and Aβ42 are valuable as biomarkers of AD. At present, their strength lies mostly in their ability to support neurodegenerative etiology criteria for MCI and AD, and their reasonable capacity to predict the conversion from MCI to AD. A combination of biomarkers seems to be more useful in prediction than a single analyte.
The below table published by Mattsson N, et al. (2012) summarizes the specificities and likelihood ratios at cutoffs for 85% sensitivity for AD dementia according to age categories. The specificity of CSF biomarkers decreases with age, as an effect of the high AD prevalence in older ages, but the likelihood ratios are improved when CSF biomarkers are combined.
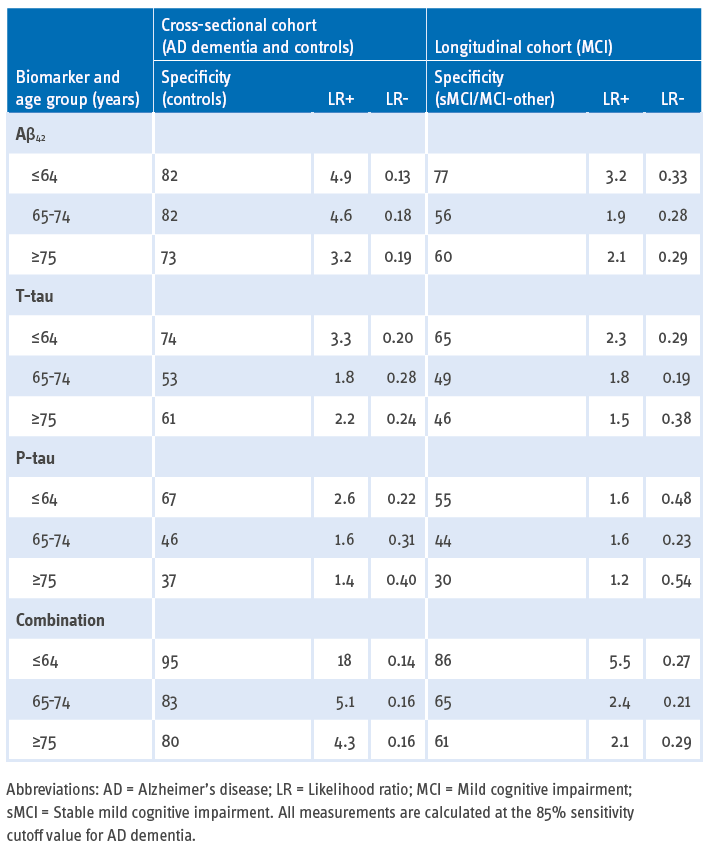
The CSF biomarkers in combination, e.g., low CSF Aβ42 peptide with high T-tau and P-tau, are sensitive and specific biomarkers highly predictive of progression to AD dementia in patients with MCI and of presence of AD etiology even in older populations.
CSF markers for AD risk stratification and predictive value
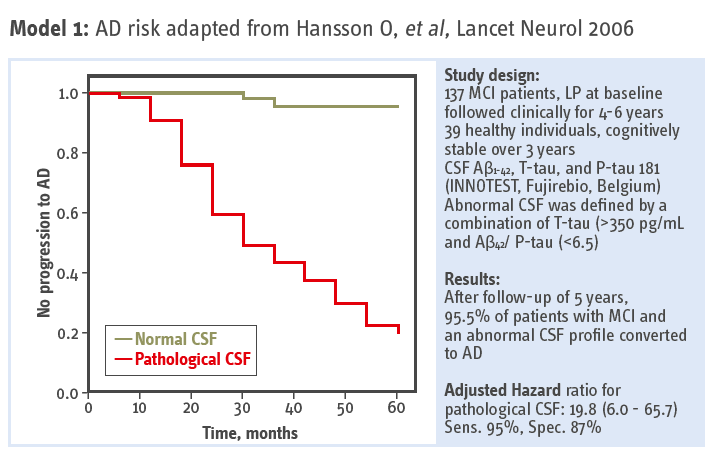
Association between CSF biomarkers and incipient AD – Monocentric longitudinal study by Hansson O, et al. (2006). The follow-up period was extended with 5-10 years and the results were published by Buchhave P, et al. (2012).
95.5 % of patients with MCI and abnormal CSF converted to AD.
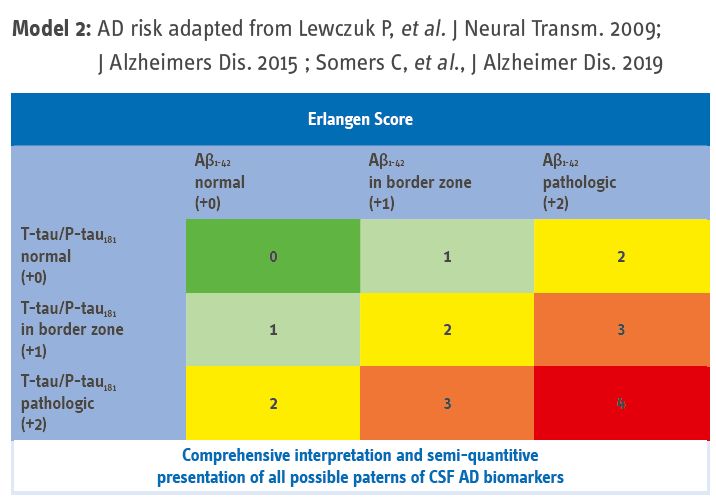
The Erlangen score was validated using two cohorts of pre-dementia subjects, the German Dementia Competence Network (n = 190 subjects with MCI) and the US Alzheimer’s Disease Neuroimaging Initiative 1 (n = 292 MCI or cognitively normal subjects). The Erlangen score uses a risk-based approach.
The CSF results of a given patient are scored between 0 and 4 points. A CSF result with all biomarkers entirely normal is scored 0 points; a pattern with only marginal alterations in one biomarkers group (either Aβ or Tau, but not both) results in the score of 1; a CSF result with the alterations in either Aβ metabolism (decreased Aβ42 concentration and/or decreased Aβ42/40 ratio) or Tau metabolism (increased concentrations of T-tau and/or P-Tau) but not both is scored 2 points; a result with clear alterations in one biomarkers’ group (either Aβ or Tau) accompanied by marginal alterations in the other group is scored 3 points; clear alterations in both Aβ and T-tau/P-Tau result in 4 points.
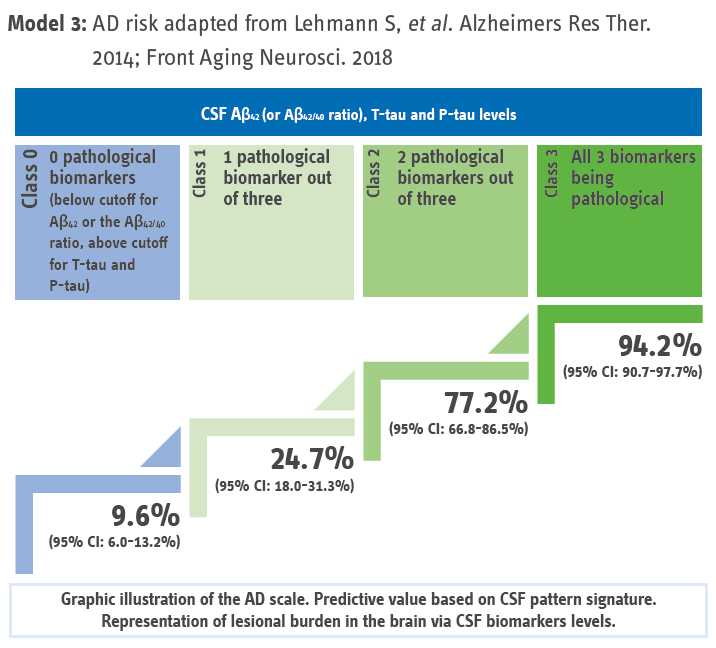
The scale’s overall predictive value for AD for the different categories (n = 1,273 patients including 646 AD and 627 non-AD) from six independent memory-clinic cohorts.
AD risk assessment may integrate the Aβ42/40 ratio (instead of Aβ42) which accounts for interindividual difference in amyloidogenic APP-processing.
These simple scales using the presence of two or three pathologic biomarkers as a criterion of AD can be used to facilitate the interpretation of CSF pattern in routine.
Remark: The two last illustrations of risk scoring are cutoff values independent, meaning each laboratory can easily supplement it with the cutoff values and normal/abnormal ranges according to the analytical method used for biomarker measurement.
CSF biosignature: a dynamic of neuropathologic changes - Not a standalone diagnostic
The combination of CSF biomarkers permits a diagnosis of AD in earlier stages of the disease. Nevertheless, the clinical identification of cognitive impairment and the use of both structural (CT/MRI) and functional (SPECT/PET) brain imaging are necessary for an accurate differential diagnosis with other neurodegenerative diseases. Mixed pathology, especially in elderly subjects, is frequent.
Bibliography
- Amyloid-β PET—Correlation with cerebrospinal fluid biomarkers and prediction of Alzheimer’s disease diagnosis in a memory clinic. Müller EG, et al. PLoS One. 2019; 14(8): e0221365. Observational Study.
- Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Dubois B, et al. Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA. Alzheimers Dement. 2016; 12(3): 292-323. Review.
- Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Blennow K, et al. Alzheimers Dement. 2015; 11(1): 58-69. Review.
- Rethinking on the concept of biomarkers in preclinical Alzheimer’s disease. Berti V, et al. Neurol Sci. 2016; 37(5): 663-672. Review.
- Interpreting Biomarker Results in Individual Patients with Mild Cognitive Impairment in the Alzheimer’s Biomarkers in Daily Practice (ABIDE) Project. van Maurik IS, et al. Alzheimer’s Disease Neuroimaging Initiative. JAMA Neurol. 2017; 74(12): 1481-1491.
- The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Glodzik-Sobanska L, et al. Neurobiol Aging. 2009; 30(5): 672-681.
- Cerebrospinal fluid biomarkers of Alzheimer’s disease in cognitively healthy elderly. Randall C, et al. Front Biosci (Landmark Ed). 2013; 18: 1150-1173. Review.
- Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. Montine TJ, et al. Neuromolecular Med. 2011; 13(1): 37-43.
- Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Paternicò D, et al. Alzheimers Dement. 2012; 8(6): 520-527. Comparative Study.
- Age and diagnostic performance of Alzheimer disease CSF biomarkers. Mattsson N, et al. Neurology. 2012; 78(7): 468-476.
- Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jansen WJ, et al. JAMA. 2015; 313(19): 1924-1938. Meta-Analysis.
- Cerebrospinal fluid biomarkers and prediction of conversion in patients with mild cognitive impairment: 4-year follow-up in a routine clinical setting. Lanari A, et al. Scientific World Journal. 2009; 9: 961-966.
- Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Hansson O, et al. Lancet Neurol. 2006; 5(3): 228-234. Comparative Study.
- Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Buchhave P, et al. Arch Gen Psychiatry. 2012; 69(1): 98-106. Comparative Study.
- A diagnostic scale for Alzheimer’s disease based on cerebrospinal fluid biomarker profiles. Lehmann S, et al. Alzheimers Res Ther. 2014; 6(3): 38.
- Relevance of Aβ42/40 Ratio for Detection of Alzheimer Disease Pathology in Clinical Routine: The PLMR Scale. Lehmann S, et al. Front Aging Neurosci. 2018; 28(10):138
- Neurochemical dementia diagnostics: a simple algorithm for interpretation of the CSF biomarkers. Lewczuk P, et al. J Neural Transm (Vienna). 2009; 116(9): 1163-1167.
- Validation of the Erlangen Score Algorithm for the Prediction of the Development of Dementia due to Alzheimer’s Disease in Pre-Dementia Subjects. Lewczuk P, et al. J Alzheimers Dis. 2015; 48(2): 433-441.
- Validation of the Erlangen Score Algorithm for Differential Dementia Diagnosis in Autopsy-Confirmed Subjects. Somers C, et al. J Alzheimers Dis. 2019; 68(3): 1151-1159.