Lumipulse® sTREM2 Controls
These controls are intended for use as an assayed quality control to monitor the precision and accuracy of laboratory testing procedures for the analysis of soluble triggering receptor expressed on myeloid cells 2 (sTREM2) in human serum, plasma and cerebrospinal fluid (CSF) on the automated LUMIPULSE® G System.
This product is not for use in diagnostic procedures, for ‘Research Use Only’.
Product number 81658
Click here to navigate
- Contact sales for information
- Documentation
- Insights
- Product inquiry
- Related products
- Webinars
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
-
Insights
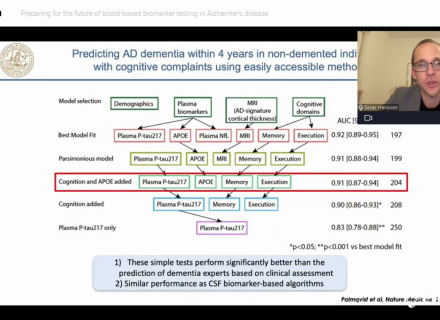
Jul 23, 2025Fujirebio introduces its Neuro Expert Toolbox (NExT) at AAIC 2025
Fujirebio is introducing its Neuro Expert Toolbox (NExT) for the first time at AAIC 2025 (Alzheimer's Association International Conference®) in...
Nov 13, 2024Video - Alzheimer's awareness redefined
Follow the journey of the Sullivan family and leading Alzheimer’s Neurologist and Researcher Dr. David Greeley as they introduce and explain these...
Feb 20, 2024Publication - Serum and cerebrospinal fluid neurofilament light chains measured by SIMOA™, Ella™, and Lumipulse™ in multiple sclerosis naïve patients
We would like to draw your attention to a first publication on our Lumipulse® G NfL solution. In this article you will find a method comparison of CSF...
-
Product inquiry
-
Related products