Testimonial - Value of the β-Amyloid ratio and other CSF biomarkers in the Erlangen Score interpretation algorithm
By Prof. Dr. Piotr Lewczuk, Professor of Neurochemistry and Head, Laboratory for Clinical Neurochemistry and Neurochemical Dementia Diagnostics, Department of Psychiatry and Psychotherapy, Universitätsklinikum Erlangen, Germany
Two groups of established cerebrospinal fluid (CSF) biomarkers reflect two major pathological alterations in Alzheimer's disease (AD): accumulation of amyloid β (Aβ) peptides in the brain parenchyma and neurodegeneration process accompanied by intracellular deposition of neurofibrillary tangles composed of molecules of (hyperphosphorylated) Tau.
Decreased concentrations of Aβ peptides ending at the C-terminus of 42 (Aβ42) and/or decreased ratio of Aβ42/40, along with elevated CSF levels of Tau and/or pTau represent the classic pattern of the CSF changes in AD.
Every human being produces Aβ peptides, by enzymatic processing of ubiquitously expressed transmembrane Amyloid Precursor Proteins (APP), beginning already in the prenatal stage and until death. The physiological role of the Aβ peptides is still unclear, but it is astonishing that an adult subject without Aβ peptides in the CSF has never been reported, which might lead to a speculation that they are - in some way - crucially important to survive.
Releasing of Aβ peptides from APP is an entirely physiological process. Pathology starts only then when, for still unclear reasons, Aβ monomers begin to accumulate to form extremely neurotoxic soluble oligomers.
When the process continues, oligomers turn into longer forms, fibrils, which, at some moment, cease to be soluble and start accumulating into plaques. A local neuroinflammation appears around the plaques followed by occurrence of neurodegeneration. Dying neurons release Tau molecules, along with their hyperphosphorylated forms, probably when the capacities of the brain to sustain accumulation of Aβ peptides are exceeded.
It takes approximately 20 years from the onset of the pathological processes (oligomerization of Aβ monomers) to the first clinically visible symptoms, i.e. the stage of mild cognitive impairment (MCI). This means that we are able to diagnose (or at least suspect) AD with CSF biomarkers about two decades before the onset of the clinical symptoms.
Twenty years ago, the Lab for Clinical Neurochemistry in Erlangen (Germany) was the first center which established the CSF Aβ42/40 concentration ratio as a routine diagnostic biomarker in AD, with inspiring ideas coming from different directions.
Since then, dozens of studies have reconfirmed improved performance of the Aβ42/40 ratio, compared to the Aβ42 concentration alone, as a diagnostic and prognostic biomarker in AD. Consequently, around 2015 several centers worldwide started using the Aβ42/40 ratio as a routine diagnostic tool.
Considering that it is only the numerator of the quotient (concentration of Aβ42) which is altered in AD, with the denominator (concentration of Aβ40) remaining practically unchanged, a question appears why normalizing the Aβ42 concentration to the concentration of the most abundant Aβ peptide, Aβ40, leads to a much better separation of AD and non-AD subjects.
A more elaborated answer to this question requires analysis of the distribution of a ratio of two stochastically proportional random variables and the relations of their measures of dispersion (variances).
This leads to a mathematically-proven conclusion that better diagnostic Aβ42/40 performance is backed up by fundamental laws of probability, which we present elsewhere[1] and can be summarized as follows: consider three groups of persons, whose Aβ42 and Aβ40 concentrations follow some distributions (as a matter of fact, at least for Aβ40, it was shown that this distribution is almost perfectly normal).
This means that under physiological conditions, a great majority of the population has average concentrations of Aβ42 and Aβ40. However, there exist persons with either very high (at the right tail of the distribution) or very low (at the left side of the distribution) physiologic concentrations of Aβ peptides.
When a person from the middle part of the distribution develops AD, his Aβ42 concentration decreases and ends up below some laboratory’s reference range; this leads to a rather clear interpretation (Fig. 1, left part).
Also, a person with high concentrations of the two peptides (right tail of the distribution) will have, under condition of AD, a decreased Aβ42 concentration, which, however, will remain relatively high due to a high starting concentration; hence it will remain above the laboratory cut-off (Fig. 1, middle part). Based on Aβ42 alone, not knowing the starting level (which we never know) and neglecting Aβ40 this will lead to a false-negative interpretation.
Figure 1:
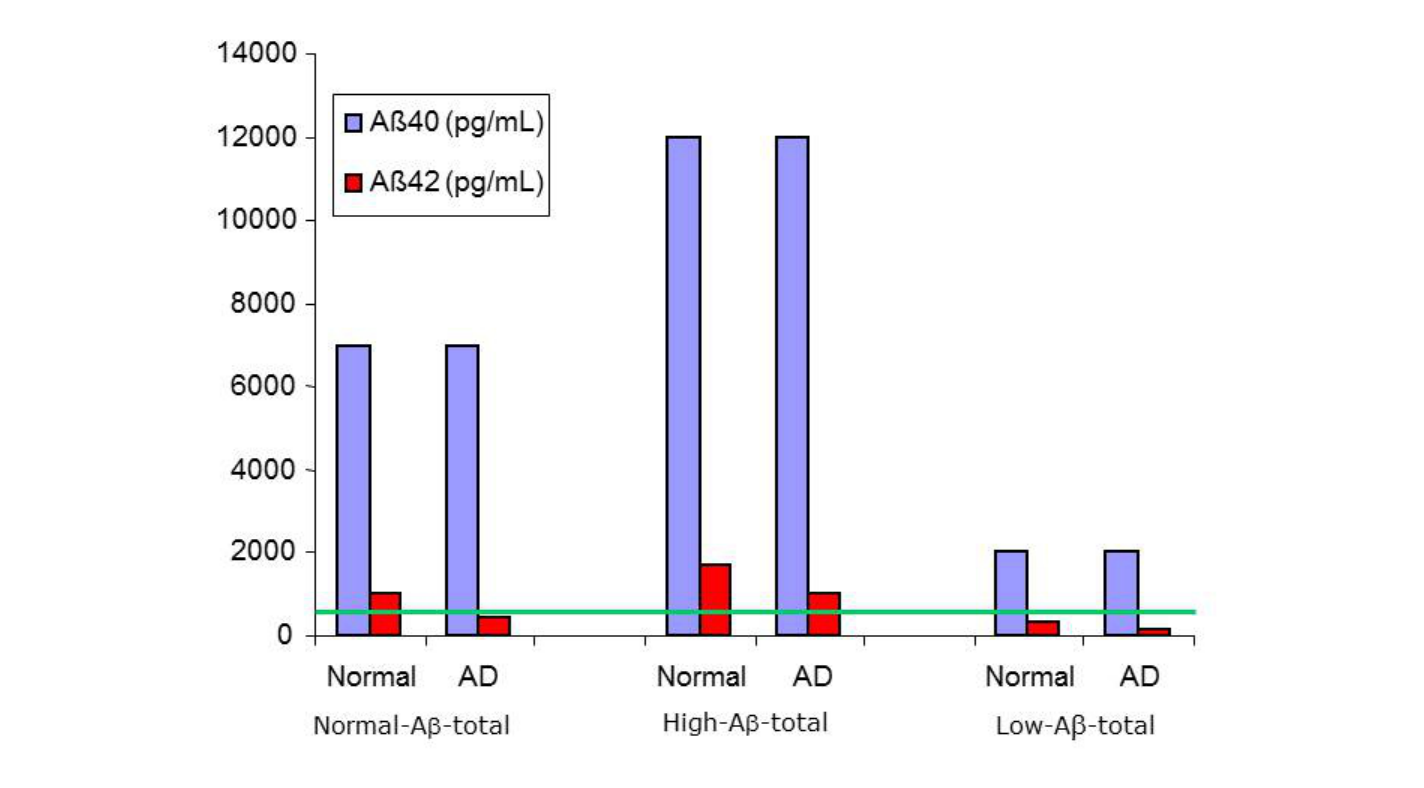
But an even worse situation occurs in the subjects from the lower (left tail) side of the Aβ distribution. Their Aβ42 concentration, already under physiologic conditions, is so low that we would (biochemically) diagnose them as AD patients, with all possible socio-medical consequences.
The error we make in this approach is due to the application of cross-sectional laboratory reference ranges to analyze longitudinal evolution of the biomarkers (a similar mistake is often made when so-called baby growth percentile curves are used to infer whether development of a given child remains in a "proper channel").
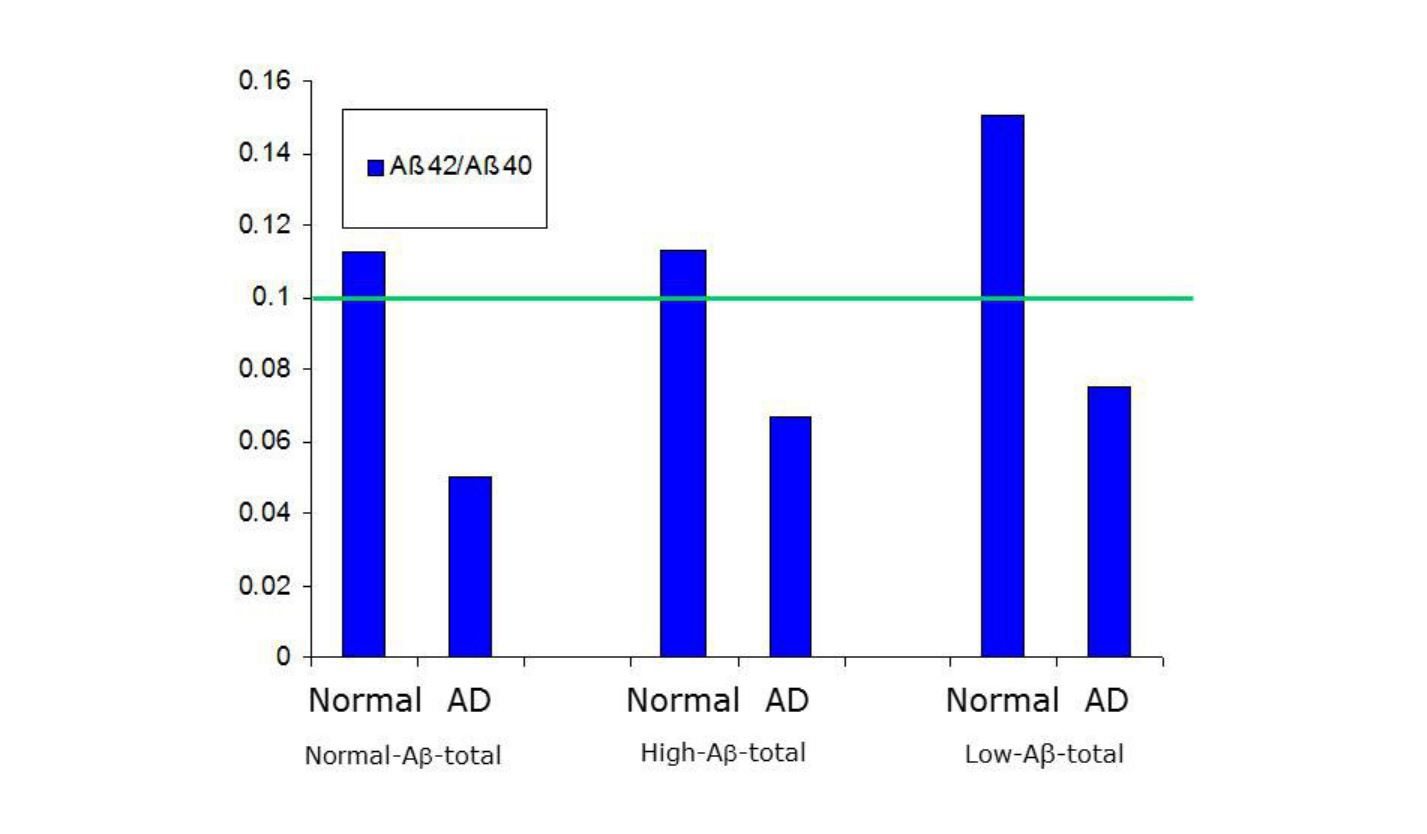
In all three situations described above, what corresponds to the real (patho)physiological situation, is the Aβ42/40 ratio (Fig. 2).
Figure 2:
The two groups of the CSF biomarkers show high diagnostic accuracy, and are routinely used as an AD diagnostics tool in many countries. However, their global acceptance is hampered because of lack of comparability of the results generated in different laboratories, for example due to different analytical platforms used.
This has already been addressed, to some extent, by efforts to standardize procedures for collection of samples, assays calibrators, and measurements protocols (including automation); however, the global acceptance of these novel approaches will certainly need time.
In addition, as the AD CSF biomarkers are more often measured in the clinical routine, interpretation of these results require some expertise. It is rarely the case that the CSF AD biomarkers fall into clear-cut normal/abnormal categories, particularly at very early stages of the disease.
In order to harmonize the diagnostic-oriented interpretation of the CSF profiles, the Erlangen Score (ES) interpretation algorithm was proposed and extensively validated on the ground of well-characterized patient cohorts from European and US centers.
According to the Erlangen Score, a result of the CSF analysis with all biomarkers normal is scored with 0 points, and interpreted as "no neurochemical evidence for AD"; a pattern with border zone changes in one biomarkers group (either Aβ or Tau/pTau, but not both) results in the score of 1, and is reported as "neurochemically improbable AD"; a CSF result with evident alterations in either Aβ metabolism (decreased Aβ42 concentration or Aβ42/Aβ40 ratio) or tau metabolism (increased Tau levels and/or pTau181) but not both is scored 2 points; the same score is given in the case of border zone alterations in the CSF biomarkers of both groups.
A result with evident changes in one biomarkers' group (either Aβ or Tau) accompanied by border zone alterations in the other group is scored 3 points; these two cases (with the ES = 2 or 3) are interpreted as "neurochemically possible AD".
Evident changes in both Aβ and Tau groups result in 4 points, and are interpreted as "neurochemically probable AD".
Isolated, very high Tau concentration is reported as suspected rapidly progressing neurodegeneration with improbable AD.
The Erlangen Score pattern can be summarized and reported to clinicians in a graphical form as presented in Fig. 3 (originally published in [2]; © 2017 the Authors).
Figure 3:
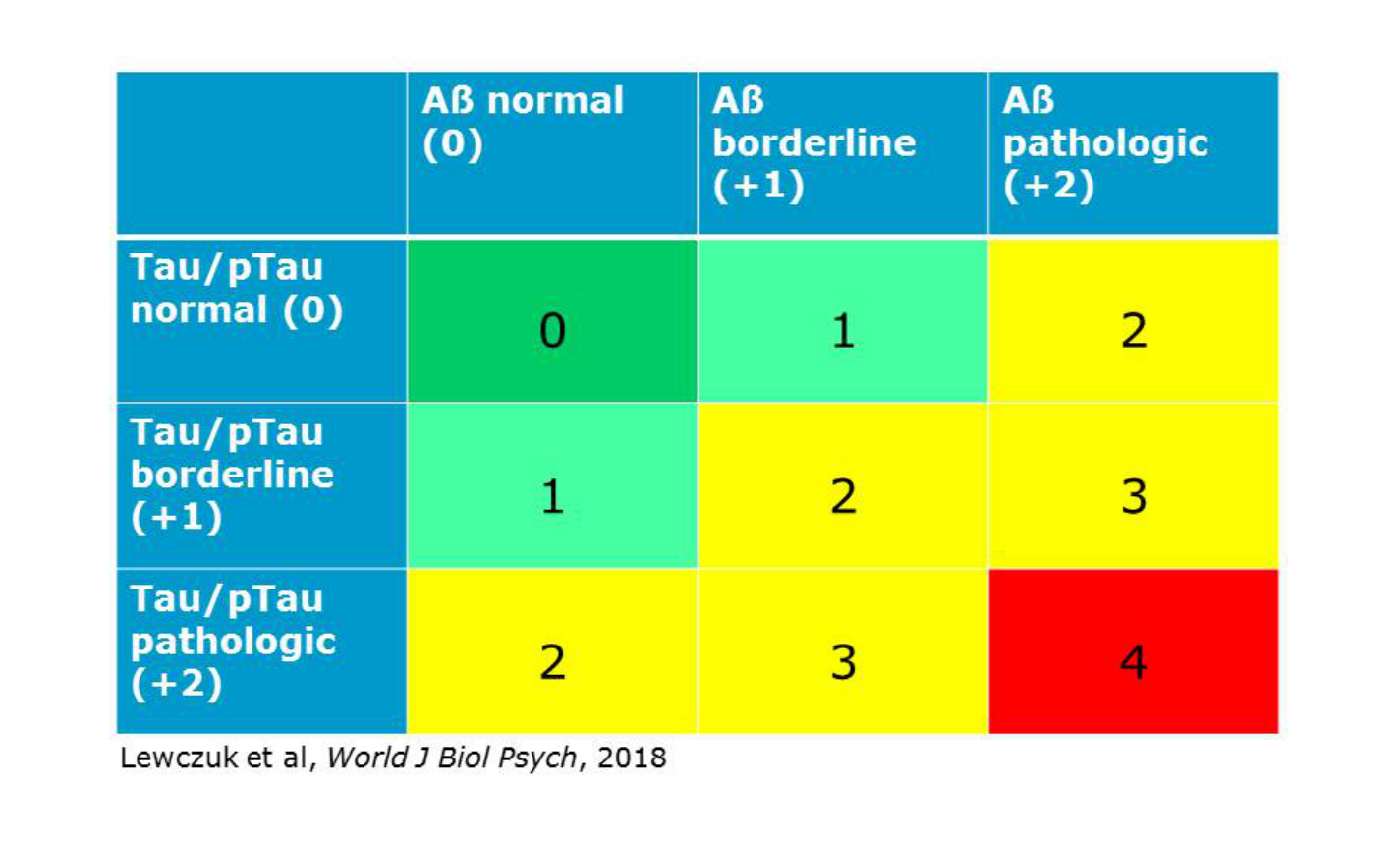
One of the key concepts in the ES algorithm are the "border zone" results. They need to be defined by each laboratory, with a suggested value of about 10%.
Irrespective of the magnitude, however, it is important to note that border zones of the ES are asymmetric; they exclusively affect results on the pathologic side of the reference value. For example, with the reference value of Aβ42/40 ratio in Erlangen laboratory being 0.06, all results above this cut off are considered entirely normal, but results within 0.055 - 0.06 are interpreted as “borderline pathologic”.
Similarly, results of Tau concentrations lower than 415 pg/mL are considered normal, but results within 415 - 455 pg/mL are considered borderline pathologic. Such asymmetric interpretation has ethical reasons; we believe it is ethically more correct to underdiagnose patients, by accepting a higher percentage of false negative results, than to overdiagnose (by making more false positive errors).
ES shows clear advantages compared to other interpretational approaches. It enables categorization of the CSF results into five classes on an ordinal scale (0 - 4), with an increasing level of pathological changes of the CSF biomarkers in AD.
Furthermore, it includes, for the first time in the interpretation of the AD biomarkers in CSF, the idea of the border zone results.
This is clearly opposite to a dichotomous approach (CSF normal/pathologic) applied in earlier interpretations.
In addition, ES is less complicated and more robust in comparison to regression-based approaches or decision trees; for use in every-day laboratory routine in the Lab for Clinical Neurochemistry in Erlangen it has been fully automated, including graphical representation, with just a dozen lines of programming code.
Compared to the otherwise very reliable A/T/N classification[3], ES stratifies individuals into classes on an ordinal scale, and not into entirely descriptive categories, which enables at least semi-quantitative correlation of the CSF findings with other metrics (such as odds ratios, MCI-to-dementia progression hazards, or quantitative results of neuroimaging).
Finally, and perhaps most importantly, ES is very flexible, enabling inclusion of further potentially interesting biomarkers (as long as they reflect amyloid pathology or neurodegeneration at least on an ordinal scale) without the necessity to recalculate the number of categories. This makes ES different from other published algorithms, e.g. the Paris-Lyon-Montpellier Scale[4] based on the number of the pathologic CSF biomarkers. Irrespective of the number of the biomarkers considered, the ES will always classify the CSF patterns into five ordinal categories.
References
- Lewczuk et al., Diagnostics (Basel), 2021, 11(12): 2372.
- Lewczuk et al., World J Biol Psychiatry, 2018, 19(4): 244-328.
- Jack et al., Alzheimers Dement, 2018, 14(4): 535-562.
- Lehmann et al., Front Aging Neurosci, 2018, 10:138.