Improving clinical diagnosis of Alzheimer’s disease: Review of the available literature
By Rianne Esquivel and Dr. Nathalie Le Bastard, Fujirebio
- You can download this entire article as a PDF here (Premium eServices resource)
In Part 1 of this article, we discussed the drawbacks of relying solely on the standard routine clinical examinations and cognitive testing for making a diagnosis of Alzheimer’s disease (AD).
In this chapter, we will review available literature on the accuracy of the underlying pathological determinations in mild cognitive impairment (MCI) and non-AD patients during clinical diagnosis and how amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) testing can influence an accurate amyloid status determination.
Amyloid PET tracers have been approved by the Food and Drug Administration and European Medicines Agency for use in assessing amyloid pathology and have published sensitivities and specificities ranging from 82% - 98% and 77% - 95%, respectively (Package Inserts: Amyvid, florbetapir [18F]; Vizamyl, flutemetamol [18F]; Neuraceq, florbetaben [18F]).
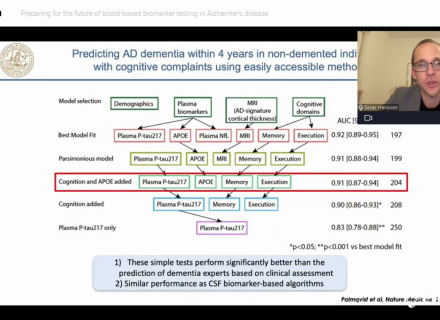
Analysis of CSF from MCI patients to determine amyloid pathology has been compared to amyloid PET as well as autopsy to determine sensitivity and specificity. In a study by Palmqvist et al. (2014), CSF β-amyloid1-42 (Aβ1-42) and amyloid imaging using [18F]flutemetamol PET were compared in patients with MCI from the Swedish BioFINDER study. CSF Aβ1-42, measured as part of routine clinical practice showed high accuracy for determining cortical Aβ levels in MCI patients, with 92% of patients identically classified (Palmqvist et al., 2014).
Utilizing the Aβ1-42/Aβ1-40 ratio which normalizes the measurement of Aβ1-42 to the individual patient’s amyloid production increases sensitivity and specificity (Hansson et al., 2019). In a study of 100 MCI patients, the CSF Aβ1-42 concentration could distinguish individuals with an abnormal amyloid PET scan achieving a sensitivity of 82% and a specificity of 81%, while the CSF Aβ1-42/Aβ1-40 ratio achieved 96% and 91%, respectively (Pannee et al., 2016).
CSF and PET have not been extensively examined with autopsy-confirmed patients as a predictor of AD from MCI.
In a head-to-head comparison of PET and CSF in 122 healthy elderly and 34 patients with MCI who developed AD dementia within 3 years (MCI-AD), CSF Aβ1-42/tTau had the highest accuracy with a sensitivity of 97%, and specificity of 83%. PET had a similarly high accuracy for incipient AD with a sensitivity of 85% and specificity of 87%. The combination of CSF and PET was not better than using either biomarker separately (Palmqvist et al., 2015).
In an additional CSF study, 37 MCI subjects who converted to a clinical diagnosis of probable AD within one year were shown to have significantly lower Aβ1-42 levels than the cognitively normal comparators (Shaw et al., 2009); and in a study looking at 137 MCI patients who were followed for 4 to 6 years, 57 developed AD, 21 developed other forms of dementia, and 56 remained cognitively stable. A ratio result combining CSF tTau and Aβ1-42 at baseline yielded a sensitivity of 95% and a specificity of 83% for detection of incipient AD in patients with MCI (Hansson et al., 2006).
Differentiating AD from non-AD dementia is also challenging when utilizing cognitive screening tests alone and can be improved by the use of CSF biomarkers.
A combination of Aβ1-42, tTau and pTau was evaluated for distinguishing autopsy-confirmed AD (n=61) from frontotemporal lobar degeneration (FTLD; n=14). Whereas clinical evaluation had a sensitivity and specificity of 80%, a regression model including CSF Aβ1-42 and pTau had a sensitivity of 98% and specificity of 93%. CSF testing improved accuracy compared to clinical evaluation by recategorizing participants with autopsy-confirmed AD who had been incorrectly clinically classified as having FTLD. CSF testing also corrected a small number of false positives and incorrectly recategorized a small number of true positives from clinical evaluation.
Interestingly this study included 27 patients diagnosed at MCI at the time of CSF sampling and found no difference in the accuracy of CSF biomarkers at the MCI versus dementia stage (Toledo et al., 2012; Fink et al., 2020).
In an additional study of AD and FTLD patients the sensitivity and specificity of clinical evaluation to distinguish between autopsy-confirmed AD (n=20) and FTLD (n=10) was 67% and 87%, respectively, whereas the tTau/Aβ1-42 ratio, sensitivity and specificity was 90% and 97% respectively (Irwin et al., 2012).
Finally, in a study including 80 AD and 75 non-AD patients, a large proportion of which had an autopsy-confirmed diagnosis (73/80 AD and 38/75 non-AD), a subset of 16 out of 111 patients could not be discriminated when using clinical evaluation alone. A sequential decision tree that included pTau and Aβ1-42/Aβ1-40, correctly classified 7 of the 16 individuals, while a decision tree that also included Aβ1-40 correctly categorized 10 of the 16 (p=0.30; calculated by review authors) (Slaets et al., 2013; Fink et al., 2020).
An additional two studies looked at amyloid PET imaging and clinical diagnosis in patients with autopsy confirmation.
In the first of these two studies that looked at 59 patients ranging from normal to dementia status, clinical evaluation had sensitivity of 72% (28/39) and specificity of 95% for neuropathologically confirmed AD. Amyloid PET corrected 10 of 11 clinical false negatives and one clinical false positive, but miscategorized 2 of 28 clinical true positives (Clark et al., 2012; Fink et al., 2020).
In the second study that included 57 patients with a clinical diagnosis of AD, 3 with dementia with Lewy bodies (DLB), 6 with other dementias, and 8 without dementia clinical evaluation had sensitivity of 94% (44/47) and specificity of 52% (14/27) whereas amyloid PET had sensitivity of 98% and specificity of 89% (Sabri et al., 2015).
CSF measurements or amyloid PET alongside clinical diagnosis provides greater accuracy in determining amyloid status than clinical diagnosis with only cognitive screening tests. This is especially important in differentiating AD from non-AD dementia and in MCI patients who are often misdiagnosed with MCI due to AD.
It is well known that cholinesterase inhibitors prescribed for AD patients have adverse interactions in FTLD patients, with worsening of cognitive and behavioral symptoms (Noufi et al., 2019).
Similarly, antipsychotic medications used to treat symptoms of AD can cause neuroleptic sensitivity in as many as 81% of patients with DLB (Armstrong & Weintraub, 2016) and has therefore become a suggestive feature in the DLB clinical diagnostic criteria (McKeith et al., 2020).
Finally, with the groundbreaking approval of the first disease-modifying drug for AD in the US, AduhelmTM, a drug that targets amyloid deposition, it is critically important to correctly identify MCI patients with amyloid pathology. This will ensure a better determination of which patients may benefit from the drug, without exposing patients who do not have AD to unwanted side effects.
As discussed in this review, clinical diagnosis with cognitive screening tests, like the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment (MoCA), alone does not ensure a correct pathological diagnosis especially in MCI patients. Moving forward it will be important to consider PET or CSF results to determine underlying pathology in patients with MCI that is persistent, progressing, and unexplained.
References
- Armstrong, M.J., Weintraub, D. (2016). The Case for Antipsychotics in Dementia with Lewy Bodies. Mov Disord Clin Pract, 4(1), 32-35. https://doi.org/10.1002/mdc3.12383
- Clark, C. M., Pontecorvo, M. J., Beach, T. G., Bedell, B. J., Coleman, R. E., Doraiswamy, P. M., Fleisher, A. S., Reiman, E. M., Sabbagh, M. N., Sadowsky, C. H., Schneider, J. A., Arora, A., Carpenter, A. P., Flitter, M. L., Joshi, A. D., Krautkramer, M. J., Lu, M., Mintun, M. A., & Skovronsky, D. M. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. The Lancet Neurology, 11(8), 669–678. https://doi.org/10.1016/S1474-4422(12)70142-4
- Fink, H. A., Hemmy, L. S., Linskens, E. J., Silverman, P. C., MacDonald, R., McCarten, J. R., Talley, K. M. C., Desai, P. J., Forte, M. L., Miller, M. A., Brasure, M., Nelson, V. A., Taylor, B. C., Ng, W., Ouellette, J. M., Greer, N. L., Sheets, K. M., Wilt, T. J., & Butler, M. (2020). Key Question 2: Biomarkers for Identifying Neuropathologically Confirmed AD. In: “Diagnosis and Treatment of Clinical Alzheimer’s-Type Dementia: A Systematic Review” https://www.ncbi.nlm.nih.gov/books/NBK556551/
- Hansson, O., Lehmann, S., Otto, M., Zetterberg, H., & Lewczuk, P. (2019). Advantages and disadvantages of the use of the CSF Amyloid beta (Abeta) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimer’s Research & Therapy, 11(1), 34. https://doi.org/10.1186/s13195-019-0485-0
- Hansson, O., Zetterberg, H., Buchhave, P., Londos, E., Blennow, K., & Minthon, L. (2006). Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. The Lancet. Neurology, 5(3), 228–234. https://doi.org/10.1016/S1474-4422(06)70355-6
- Irwin, D. J., McMillan, C. T., Toledo, J. B., Arnold, S. E., Shaw, L. M., Wang, L.-S., van Deerlin, V., Lee, V. M.-Y., Trojanowski, J. Q., & Grossman, M. (2012). Comparison of cerebrospinal fluid levels of tau and Abeta 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Archives of Neurology, 69(8), 1018–1025. https://doi.org/10.1001/archneurol.2012.26
- McKeith, I. G., Ferman, T. J., Thomas, A. J., Blanc, F., Boeve, B. F., Fujishiro, H., Kantarci, K., Muscio, C., O’Brien, J. T., Postuma, R. B., Aarsland, D., Ballard, C., Bonanni, L., Donaghy, P., Emre, M., Galvin, J. E., Galasko, D., Goldman, J. G., Gomperts, S. N., … Tiraboschi, P., for the prodromal DLB Diagnostics Study Group (2020). Research criteria for the diagnosis of prodromal dementia with Lewy bodies. In Neurology, 94(17), 743–755. Lippincott Williams and Wilkins. https://doi.org/10.1212/WNL.0000000000009323
- Noufi, P., Khoury, R., Jeyakumar, S., & Grossberg, G. T. (2019). Use of Cholinesterase Inhibitors in Non-Alzheimer’s Dementias. In Drugs and Aging (Vol. 36, Issue 8, pp. 719–731). Springer International Publishing. https://doi.org/10.1007/s40266-019-00685-6
- Palmqvist, S., Zetterberg, H., Blennow, K., Vestberg, S., Andreasson, U., Brooks, D. J., Owenius, R., Hägerström, D., Wollmer, P., Minthon, L., & Hansson, O. (2014). Accuracy of Brain Amyloid Detection in Clinical Practice Using Cerebrospinal Fluid β-Amyloid 42. JAMA Neurology, 71(10), 1282. https://doi.org/10.1001/jamaneurol.2014.1358
- Palmqvist, S., Zetterberg, H., Mattson, N., Johansson, P., Alzheimer’s Disease Neuroimaging Initiative, Minthon, L., Blennow K., Olsson, M., Hansson, O., Swedish BioFINDER Study Group. (2015). Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology, 84(14):1240-1249. https://doi.org/ 10.1212/WNL.0000000000001991
- Pannee, J., Portelius, E., Minthon, L., Gobom, J., Andreasson, U., Zetterberg, H., Hansson, O., & Blennow, K. (2016). Reference measurement procedure for CSF Aβ 1-42 and the CSF Aβ 1-42 /Aβ 1-40 ratio - a cross-validation study against Amyloid PET. Journal of Neurochemistry. https://doi.org/10.1111/jnc.13838
- Sabri, O., Sabbagh, M. N., Seibyl, J., Barthel, H., Akatsu, H., Ouchi, Y., Senda, K., Murayama, S., Ishii, K., Takao, M., Beach, T. G., Rowe, C. C., Leverenz, J. B., Ghetti, B., Ironside, J. W., Catafau, A. M., Stephens, A. W., Mueller, A., Koglin, N., … Schulz-Schaeffer, W. J. (2015). Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimer’s and Dementia, 11(8), 964–974. https://doi.org/10.1016/j.jalz.2015.02.004
- Shaw, L. M., Vanderstichele, H., Knapik-Czajka, M., Clark, C. M., Aisen, P. S., Petersen, R. C., Blennow, K., Soares, H., Simon, A., Lewczuk, P., Dean, R., Siemers, E., Potter, W., Lee, V. M. Y., & Trojanowski, J. Q. (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of Neurology, 65(4), 403–413. https://doi.org/10.1002/ana.21610
- Slaets, S., Le Bastard, N., Martin, J.-J., Sleegers, K., van Broeckhoven, C., de Deyn, P. P., & Engelborghs, S. (2013). Cerebrospinal fluid Abeta1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. Journal of Alzheimer’s Disease, 36(4), 759–767. https://doi.org/10.3233/JAD-130107
- Toledo, J. B., Brettschneider, J., Grossman, M., Arnold, S. E., Hu, W. T., Xie, S. X., Lee, V. M. Y., Shaw, L. M., & Trojanowski, J. Q. (2012). CSF biomarkers cutoffs: The importance of coincident neuropathological diseases. Acta Neuropathologica, 124(1), 23–35. https://doi.org/10.1007/s00401-012-0983-7