New trends in cervical cancer screening: Self-sampling would allow full molecular screening – great opportunity to increase attendance to cervical cancer screening
By Rebecca Millecamps, Fujirebio
Cervical cancer screening is evolving, and one of the most promising innovations is full molecular self-screening. This method combines HPV testing with advanced molecular triage—specifically, HPV genotyping and/or DNA methylation testing—on self-collected cervico-vaginal samples. It offers a more accurate, convenient, and empowering way for women to participate in screening, especially in areas where traditional methods fall short.
Why Self-Sampling Matters
Self-sampling allows women to collect their own samples at home, which are then tested using PCR-based HPV tests. Self-collected cervico-vaginal samples have shown diagnostic performance comparable to clinician-collected samples when analyzed using PCR-based HPV tests. Countries like the Netherlands, Sweden, and Denmark have already adopted this approach in their national programs. The real advantage is that both the initial HPV test and the follow-up triage can be done on the same sample, making the process simpler and more efficient, with a lower risk of losing women in follow-up.
The Role of Genotyping and Methylation
HPV 16 and 18 genotyping plays a central role in triage, as these types are most strongly associated with progression to CIN3+. Women testing positive for these genotypes are typically referred directly for colposcopy, yet over-referral remains high. For other high-risk types, extended genotyping (HPV16/18, 31, 33, 45, 51, 52, 58) and methylation testing can help determine who truly needs follow-up, reducing unnecessary procedures while maintaining safety.
What is Methylation Testing?
Methylation testing looks for specific changes in DNA—called epigenetic changes—that occur when HPV infections persist and begin to cause high-grade lesions or cancer. These changes can be found in both the virus and the host’s DNA. Based on the methylation level of specific host cell genes, such as FAM19A4/miR124-2 and ZNF582, CIN2/3 lesions likely to regress can be distinguished from those with a high cancer progression risk (so-called “advanced” CIN lesions”). Studies with the FAM19A4/miR124-2 methylation test, including a prospective follow-up study of untreated CIN2/3 lesions (CONCERVE) and a 14-year longitudinal study, have shown that methylation testing offers a safe clinical performance, detecting ≥98% cervical cancers and showing a Negative Predictive Value (NPV) for CIN3+ equal to a negative cytology test. Methylation testing is especially useful when traditional triage with cytology (Pap smears) isn’t possible, such as with self-collected samples.
Benefits and Challenges
Methylation testing is objective, reproducible, and can be automated, making it ideal for large-scale screening. It supports a “one-sample” strategy, improving follow-up and reducing clinic visits. Importantly, studies show that a negative methylation test offers long-term reassurance with low risks of developing serious lesions, similar to a negative cytology result.
HPV testing with methylation-based triage is an ideal tool to monitor the risk of CIN3+ in HPV-vaccinated women because methylation is independent of HPV genotyping.
However, challenges remain. Clinical validation guidelines for triage methods are needed, along with implementing methylation testing in labs and clinics.
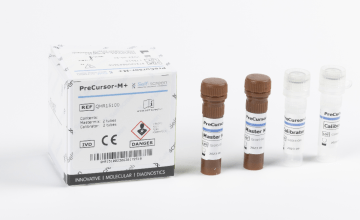
To expand the implementation of methylation testing and improve accessibility of the FAM19A4/miR124-2 methylation test, the PreCursor-M+ methylation test can be performed not only on the MIC qPCR cycler (BMS) but also on the CFX Touch cycler (Bio-Rad). Self-screen has successfully completed the validation, and this application is now also available through Fujirebio.2,3
Conclusion
Methylation testing is a game-changer in cervical cancer prevention. In combination with self-sampling, healthcare systems can offer women a more accurate, and more accessible screening — bringing us closer to the global goal of eliminating cervical cancer.
References:
- C JLM Meijer et al. (April 2025). Full molecular self-screening for cervical cancer detection: more independence, convenience and privacy for participating women - Part1 (no 292) and Part 2 (no 293). www.HPVWorld.com
- IFU PreCursor-M+ for use with Mic qPCR cycler
- IFU PreCursor-M+ for use with CFX PCR system