EUROGIN 2023 - Shared challenges to the implementation of HPV screening and diagnostics from research to practice
By Rebecca Millecamps, Fujirebio
The keynote lectures by prominent experts in their respective fields at the recent Eurogin 2023 Conference in Bilbao, Spain, once again gave a good indication of the essential issues around HPV diseases, cancer screening, and prevention. The following is a short summary of the key trends of this year's exciting conference.
Implementation of HPV-based cervical cancer screening continues
Today, extensive evidence shows the advantages of HPV primary screening versus cytology-based screening.1 At the same time, benefits and challenges should be weighed against each other, and most optimal algorithms should be established and evaluated to answer country, region, or population specific needs. The impact of vaccination should also be taken into account and extra attention should be given to vulnerable populations (e.g. immunosuppressed people, people living with HIV, MSM, as well as low income countries and young women at the child-bearing age).
Maximize the benefits while decreasing potential harm
Further optimization and improvement of cervical cancer screening is needed to maximize its benefits while decreasing the potential harm, such as cost of unnecessary colposcopy referrals and treatments.
The role of methylation testing and/or HPV genotyping as prognostic biomarkers for cervical HSIL progression and triage tools for cervical cancer screening have been discussed extensively. The increased use of self-sampling and the greater emergence of immunised populations in screening were also covered at this year's conference next to the importance of clinical validation of HPV tests.
The impact of HPV vaccination and prevalence of HPV genotypes are important factors to keep in mind when looking towards the future and keeping an eye on the effectiveness of screening programs.
A need for better triage and risk stratification of hrHPV positive women
The implementation of HPV-based screening increased the detection of ≥CIN2 and higher colposcopy referral rates. As CIN 2 lesions are highly heterogeneous, there is a need for better triage and risk stratification of hrHPV positive women, to guide clinicians and gynaecologists to allow a more conservative approach for treatment of women and optimize follow-up.
In the Netherlands, for example, it is recommended since 2016 to preferably not treat CIN2 during productive age. However, the actual change in number of treatments has been very limited and there is still a lot of heterogeneity in treatment. 2,3 Also in other countries there is a growing awareness and caution to apply a more conservative approach and a need for objectivity in the guidelines. This may come from molecular technologies that help clinicians to decide who to treat and whom not.
Experience with HPV (extended) genotyping and methylation were presented, showing the added value in different clinical settings. The final answer to the optimal triage strategy is not clear yet, there will still be a lot of debate and learning, sharing experiences on the different tools available. Most probably a combination of tools will be applied, as more insight and experience will become available.
A growing interest in HPV testing on self-samples
Experience in recent years from Sweden, Denmark, the Netherlands, UK, and Australia provides indications that self-sampling can increase the coverage of screening, even during COVID-19 pandemic. Several countries have already implemented self-sampling and other countries are considering a more prominent role for self-sampling in their programs. The importance of this sampling method is growing steadily.
A full molecular flow for both screening and triage offers a great benefit, when also self-sampling will be introduced. Cytology-based triage cannot be done on self-sampling.
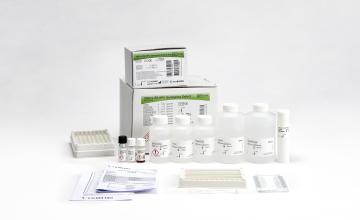
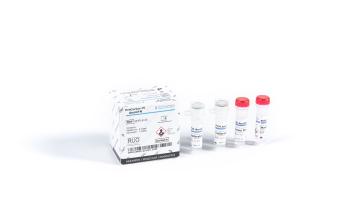
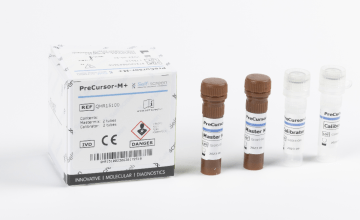
Fujirebio’s portfolio of HPV testing solutions
Triage of hrHPV positive women:
- PreCursor-M+ Assay (Self-screen B.V.): methylation-specific molecular assay detecting promotor hypermethylation of 2 cervical cancer associated markers (FAM19A4 and hsa-miR124-2)
- HPV Genotyping:
- INNO-LiPA HPV Genotying Extra II assay (Fujirebio Europe N.V.): full HPV genotyping (32 genotypes: incl. 13 HR & 6 pHR), fully automated strip processing and interpretation option available.
- AmpFire HPV High Risk Genotyping (Atila Biosystems Inc.): high risk HPV genotyping using the isothermal amplification. No need for DNA extraction. Fast, simple, accurate.
HPV screening solutions:
- AmpFire HPV Screening 16/18/HR (Atila Biosystems Inc.): isothermal amplification. No need for DNA-extraction. Fast, simple, accurate.
- HPV-Risk Assay (Self-screen B.V.): targeting E7 region. Fully clinically validated according to Meijer criteria for HPV screening.
- ScreenFire HPV Risk Stratification kit (Atila Biosystems Inc.) designed to group the carcinogenic HPV types into four channels based on clinical importance (HPV16; HPV18/45; HPV 31/33/35/52/58 and HPV 39/51/56/59/68) - only made available upon specific request
Self-sampling:
- Colli-Pee® prefilled with UCM preservative (Novosanis N.V.)
INNO-LiPA is a trademark of Fujirebio Europe N.V., registered in US and other countries.
AmpFire is a registered trademark of Atila Biosystems Inc.
PreCursor M+ and HPV-Risk Assay are registered trademarks of Self-screen B.V.
Colli-Pee is a trademark of Novosanis N.V., Belgium.
Colli-Pee prefilled with UCM preservative is distributed by Fujirebio Europe N.V; Technologipark 6 – B-9052 Gent, Belgium - Tel. +32 (0)9 329 13 29 – Fax +32 (0)9 329 19 11
References:
- P.J. Maver et al. 2020 Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect ;26:579
- Loopik DL et al. Benefit and burden in the Dutch cytology-based vs high-risk human papillomavirus-based cervical cancer screening program. Am J Obstet Gynecol 2021;224:200.e1-9.
- Aitken C.A. et al. 2019 Management and treatment of cervical intraepithelial neoplasia in the Netherlands after referral to colposcopy. Acta Obstet Gynecol Scand.;98:737–746.