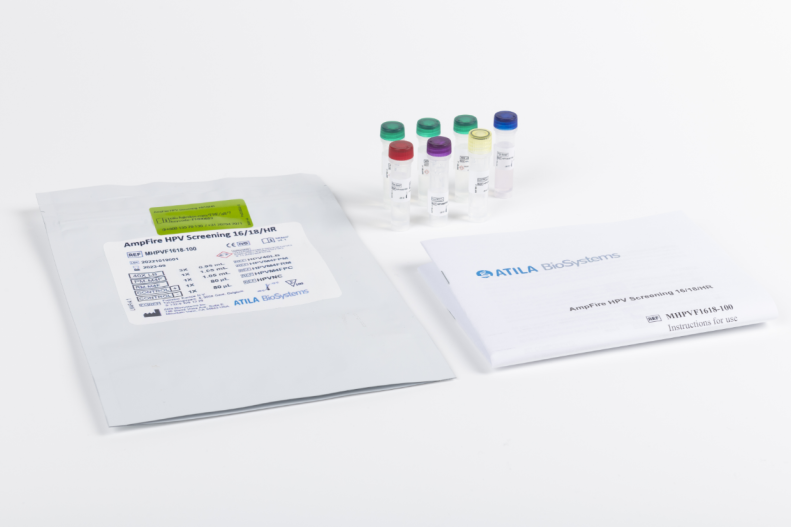
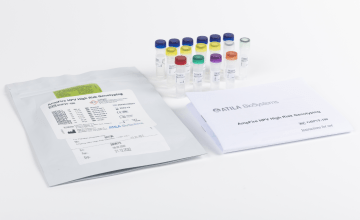
AmpFire® HPV Screening 16/18/HR
Qualitative detection of 15 High-Risk HPV types with simultaneous genotyping of HPV16 and 18 in one tube.
Fast. Simple. Accurate.
Special Features:
- Method:
- Isothermal amplification with real time fluorescence detection
- Simplicity:
- Extremely simple sample processing - no DNA purification needed
- Limited hands-on time
- Fast:
- Sample-to-result in less than 1.5 hours*
- Accurate:
- High accuracy and sensitivity with all necessary controls
- Flexible:
- Suitable for different sample types
- Flexible sample numbers per run with no waste of reagents
* depending on sample type. For FFPE samples: time to results approx. 160 minutes
Product number 80732
Click here to navigate
- Details
- Conditions of sale
- Documentation
- Insights
- Related products
- Webinars
-
Details
Technical Specifications:
- HPV types detected:
- 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68
- Sample types:
- Dry swab samples, Cervical samples stored in ThinPrep® (PreservCyt Solution), SurePathTM, FFPE biopsy, or purified DNA samples.
- Target region:
- Various regions in the viral genome including E6/E7
- Reaction volume:
- 25 µL
- Reaction time:
- Approx. 90 minutes including sample processing*
- Equipment:
- Heating block and real-time PCR system (compatible with CFX96 - Bio-Rad, ABI7500 - Thermo Fisher Scientific, Rotor-Gene Q - QIAGEN, PowerGene 9600 - Atila PCR system, and other RT-PCR systems with 5 fluorescence channels)
AmpFire is a registered trademark of Atila Biosystems Ltd., Thinprep is a registered trademark of Hologic Inc., Surepath is a registered trademark of Becton, Dickinson and Company.
- HPV types detected:
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
-
Insights
Feb 4, 2026HPV genotyping in FFPE tissue: meeting the challenges of archival sample analysis
Human papillomavirus (HPV) is a necessary cause of cervical cancer and is increasingly recognized as an important etiological factor in a range of...
Sep 24, 2025Clinical validation of the PreCursor-M AnoGYN test: Tailoring treatment of anal and vulvar cancer precursors
A major challenge for the correct implementation of the anal cancer screening guidelines is the generally limited availability of High-Resolution...
Sep 3, 2025New trends in cervical cancer screening: Self-sampling would allow full molecular screening – great opportunity to increase attendance to cervical cancer screening
Cervical cancer screening is evolving, and one of the most promising innovations is full molecular self-screening. This method combines HPV testing...
-
Related products