Can KL-6 be a specific marker of alveolar damage for COVID-19 patients?
By Victor Ruiz, Fujirebio
There are many reference centers across Europe that have included KL-6 as one of the biomarkers within the COVID-19 protocol to strengthen the battery of tests for prognostication and to allocate better the available means for invasive ventilation. Due to its critical role in reflecting the severity of lung injury and neutrophilic inflammation and its translation into clinical practice, KL-6 might become one of the revelations in laboratory medicine during these exceptional and challenging times.
- You can download a PDF version of this article here.
- Also, don't miss the resources available at www.fujirebio.com/kl-6
- Update on June 2nd 2020:
An article by d'Alessandro M, Cameli P, Refini RM, et al. "Serum KL-6 concentrations as a novel biomarker of severe COVID19" [published online ahead of print, 2020 May 29] (J Med Virol. 2020;10.1002/jmv.26087. doi:10.1002/jmv.26087) concludes that "NK cell analysis and assay of KL-6 in serum can help identify severe COVID19 patients. Increased KL-6 serum concentrations were observed in patients with severe pulmonary involvement, revealing a prognostic value and supporting the potential usefulness of KL-6 measurement to evaluate COVID19 patient prognosis." (link points to PubMed)
With the outbreak of an unknown form of pneumonia in Wuhan, China, in December 2019, a new coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) aroused the attention of the entire world.1,2
Now, 4 months later, more than half of the world's population is in compulsory or recommended confinements, curfews and quarantines to prevent the spread of the COVID-19 virus. The pandemic has become one of the worst in the last decades, confronting healthcare professionals around the world with unprecedented clinical and operational challenges.
Background
The SARS-CoV-2 is the seventh coronavirus known to infect humans and seems to replicate faster than the SAR-CoV-1 and MERS viruses. The target of the SARS-CoV-2 is confirmed to be the same cell entry receptor, angiotensin-converting enzyme 2 (ACE2), as SARS-CoV-1, which is highly expressed in airway epithelial cells, primarily on type II alveolar cell.1–3 Histological examination of infected lung tissue shows diffuse alveolar damage with cellular exudate. Signs of pneumocyte desquamation, pulmonary edema and hyaline membrane formation are present, as in cases of acute respiratory distress syndrome (ARDS).4,5 The SARS-CoV-2 infection is an acute infection with spontaneous resolution although, in some cases, it can be fatal. The clinical presentation can vary from mild respiratory symptoms to severe pneumonia with poor prognosis. A severe clinical picture at disease onset can lead to death from massive diffuse alveolar damage resulting in end-stage respiratory failure.
Clinical picture
The clinical picture (infection) associated with the SARS-CoV-2 is called COVID-19. People with COVID-19 generally develop signs and symptoms, including mild respiratory symptoms and fever, on an average of 5-6 days after infection (mean incubation period 5-6 days, range 1-14 days).6,7
The most common symptoms at onset of illness are fever, dry cough, fatigue (myalgia or asthenia) and dyspnea (shortness of breath); less common symptoms are sputum production, headache, hemoptysis (coughing up of blood), and diarrhea. In addition, rhinorrhea (nasal congestion) and sore throat can be found in some cases.6,7
From a radiological point of view, COVID-19 pneumonia shows a bilateral involvement. In more severe patients, the radiological picture often consists of lobar and sub-segmental consolidations. In less severe patients, who do not need intensive care, computerized tomography (CT) chest scan shows bilateral ground-glass opacities and areas of sub-segmental consolidation.8
Disease evolution
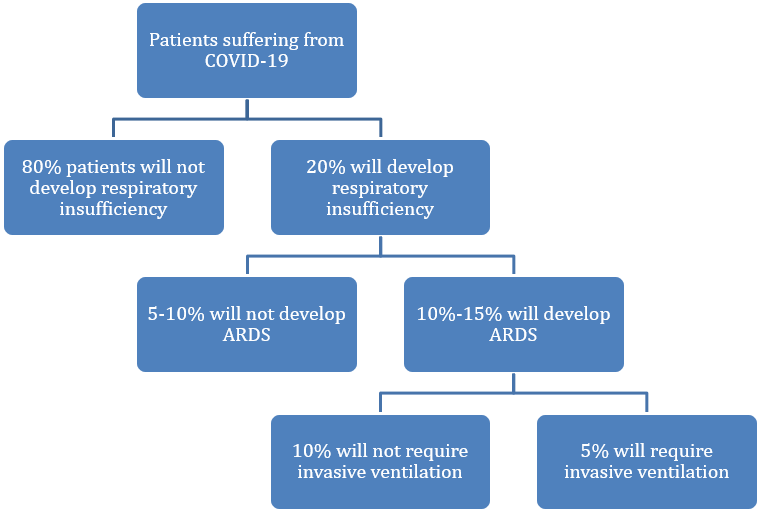
The WHO estimates that approximately 80% of laboratory confirmed patients have had mild to moderate disease, which includes non-pneumonia and pneumonia cases. The other 20% will develop respiratory insufficiency, with 13.8% having severe disease (dyspnea, respiratory frequency ≥30/minute, blood oxygen saturation ≤93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% of the lung field within 24-48 hours) and 6.1% having critical disease (respiratory failure, septic shock, and/or multiple organ dysfunction/failure).6 Within these last two groups that accounts for 20% of all the COVID-19 patients, 10-15% of the cases will develop ARDS9, requiring invasive ventilation around 5% of all patients suffering from COVID-19.
The role of laboratory medicine
Lippi and colleagues9 already address the way laboratory medicine plays a key role to counteract this viral outbreak in these exceptional times. “There are at least three major areas where in vitro diagnostics can also provide essential contributions to diagnostic reasoning and managed care of patients with suspected or confirmed SARS-CoV-2 infection. These include etiological diagnosis, patient monitoring, as well as epidemiologic surveillance.”
It is in these three areas where the in vitro diagnostics efforts are focusing:
| Diagnosis |
• Real-Time PCR • Rapid immunochromatography tests (antigen-based detection / antibody-based detection) • Serological tests in blood (IgM and IgG) |
| Staging / Prognostication / Therapeutic monitoring |
• Unspecific markers (IL-6, PCT, Ferritin, others) • Specific markers of the tissue (KL-6) |
| Epidemiologic surveillance |
• Serological tests in blood (IgM and IgG) • Rapid immunochromatography tests (antibody-based detection) |
The role of serum biomarkers have been extensively defined in many reports describing in detail the epidemiological and clinical characteristics of COVID-19 patients and how biomarkers can be of aid in staging, prognosticating and monitoring.10–17
Disease staging
There are two distinct but overlapping pathological subsets in disease evolution, the first triggered by the virus itself and the second, the host response. The acute disease progression is divided in three phases:18,19
Early infection phase
- The virus infiltrates the lung parenchyma and begins to proliferate. This stage is characterized by mild constitutional symptoms. Alveolar epithelial cells type II release inflammatory signals that mark the initial response by innate immunity (monocytes and macrophages recruitment).
- Diagnosis at this stage includes respiratory sample PCR, serum testing for SARS-CoV-2 IgM and IgG, along with chest imaging, complete blood count (CBC) and liver function tests. CBC may reveal a lymphopenia and neutrophilia. Coagulation functions and lactate dehydrogenase (LDH) are elevated and procalcitonin (PCT) might be elevated without other significant abnormalities.
Pulmonary phase
- Macrophages release cytokines that promote the inflammatory processes (vasodilation, endothelial permeability, leukocyte recruitment) and lead to fluid accumulation in the alveolus, diluting the surfactant, which triggers the onset of alveolar collapse, decreasing gas exchange and increasing the work of breathing. It is at this stage that most patients would need to be hospitalized for close observation and management. The immune response continues, neutrophils are recruited to the site of infection and release Reactive Oxygen Species (ROS) to destroy infected cells. Type I and II cells are destroyed, leading to the collapse of the alveolus and causing Acute Respiratory Distress Syndrome (ARDS). Intensive care unit admission at this time point is the norm with a percentage of patients requiring ventilators and life support if hypoxia ensues.
- Blood tests reveal increasing lymphopenia, along with transaminitis (increase alanine transaminase (ALT) and aspartate transaminase (AST)). Furthermore, markers of systemic inflammation (i.e. CRP) may be elevated, but not remarkably and PCT levels are low to normal.
Severe hyperinflammation phase
- The host inflammatory response continues to amplify (even with diminishing viral loads) and results in protein rich fluid entering the bloodstream and traveling elsewhere in the body, causing Systemic Inflammatory Response Syndrome (SIRS). The systemic inflammation (cytokine storm) may correlate with lymphopenia and is characterized by septic shock, multi-organ failure and elevation of key inflammatory markers.
- In this severe hyperinflammation phase, inflammatory cytokines and biomarkers such as interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor, macrophage inflammatory protein 1-α, tumor necrosis factor-α, C-reactive protein, ferritin, and D-dimer are significantly elevated. Troponin and N-terminal pro B-type natriuretic peptide (NT-proBNP) can also be elevated.
Specific marker of alveolar damage
Krebs von den Lungen 6 (KL-6) is a mucin-like glycoprotein expressed on the surface membrane of alveolar epithelial cells (AEC-II) and bronchiolar epithelial cells. More than 350 papers investigating the clinical significance of KL-6 in various types of Interstitial Lung Diseases (ILDs) have been published suggesting that KL-6 serum levels are useful for:20
- detecting the presence of AEC injury
- evaluating disease activity
- predicting clinical outcomes in various types of ILDs
During the infection and the subsequent immune response of COVID-19 patients, AEC-II are primarily infected and play a key role in releasing proinflammatory signals that enhance the cytokines performance. In more severe cases, accumulation of fluid in the alveolus and difficulties in breathing will be the norm. During this pulmonary phase, there will be a relevant number of cases (10-15%) in which AEC-I and AEC-II will be destroyed in great extent, leading to alveolus collapse and ARDS.
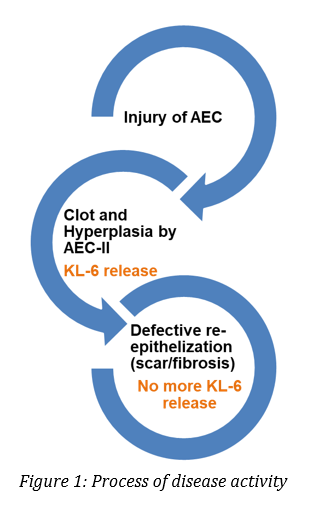
According to Koyama and colleagues21, plasma KL-6 was elevated in ARDS patients and correlated with lung injury and mortality. Besides, Kondo et al22, reported higher levels of KL-6 in non-survivors than in survivors in epithelial lining fluid (ELF) and serum samples on days 0 to 3 after ARDS diagnosis. The optimal cut-off values were set at 3453 U/mL for ELF and 530 U/mL for serum by receiver operating characteristic (ROC) curve analyses. They concluded, that patients with KL-6 concentrations in ELF higher than 3453 U/mL or serum concentrations higher than 530 U/mL had significantly lower survival rates up to 90 days after ARDS diagnosis. Further studies support the usefulness of KL-6 as a marker of alveolar type II cell dysfunction in ARDS, like Nathani et al 23, who reported that KL-6 levels reflect the severity of lung injury and neutrophilic inflammation, suggesting that KL-6 release across the alveolar epithelial barrier is associated with a poor prognosis. Data from this study showed that KL-6 levels in plasma and bronchoalveolar lavage fluid (BALF) were persistently elevated in ARDS patients compared with normal and at-risk individuals. In addition, in this patient cohort, KL-6 levels were increased in ARDS patients who subsequently died of the disease compared to those who survived.
Potential role of KL-6 in COVID-19 patients
Fujirebio Europe is committed to assist the healthcare professionals, now more than ever, to provide the best possible support for diagnosis, prognosis and therapy follow-up of COVID-19 patients. We are working side by side with physicians and laboratories to help to assess disease severity and ARDS development in critically ill patients. Our group of experts has identified two potential clinical utilities of KL-6 in patients suffering from COVID-19; first, in the stratification of ARDS patients between survivors and non-survivors (prognosis), and second, in the assessment of the residual fibrosis as a result of acute lung injury (ALI) after COVID-19 infection that many survivors will carry in the future to come.
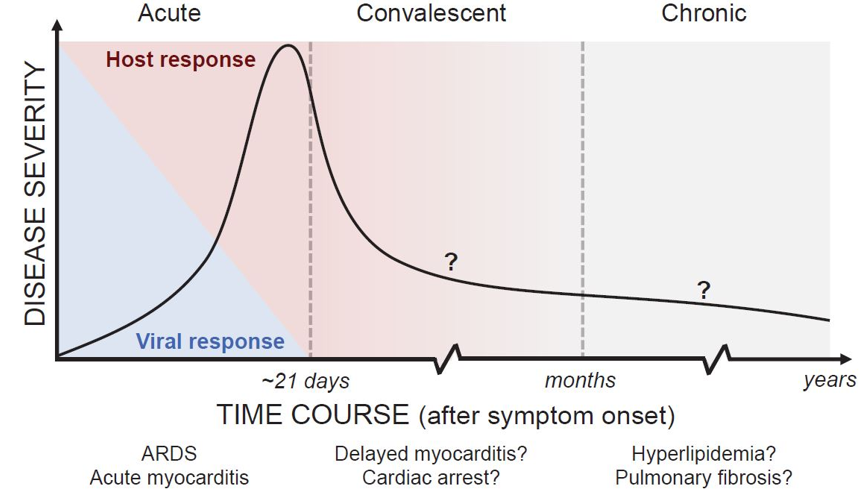
Figure 2: Classification of COVID-19 Disease States and Potential Therapeutic Targets18
- For the potential added value of KL-6 in patients suffering from pulmonary fibrosis as a result of ALI we refer to available content at www.fujirebio.com/kl-6
There are many reference centers across Europe that have included KL-6 as one of the biomarkers within the COVID-19 protocol to strengthen the battery of tests for prognostication and to allocate better the available means for invasive ventilation. Due to its critical role in reflecting the severity of lung injury and neutrophilic inflammation and its translation into clinical practice, KL-6 might become one of the revelations in laboratory medicine during these exceptional and challenging times.
References
- Zhang Y, Xu J, Li H, Cao B. A Novel Coronavirus (COVID-19) Outbreak: A Call for Action. Chest. 2020;157(4):e99-e101. doi:10.1016/j.chest.2020.02.014
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi:10.1056/NEJMoa2001017
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020:26(4):450-452. doi:10.1038/s41591-020-0820-9
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. doi:10.1016/S2213-2600(20)30076-X
- Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute respiratory distress syndrome and diffuse alveolar damage new insights on a complex relationship. Ann Am Thorac Soc. 2017;14(6):844-850. doi:10.1513/AnnalsATS.201609-728PS
- Aylward, Bruce (WHO); Liang W (PRC). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). WHO-China Jt Mission Coronavirus Dis 2019. 2020;2019(February):16-24. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mi….
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Harari SA, et al. Managing the Respiratory care of patients with COVID-19: Italian Thoracic Society and Italian Respiratory Society. 2020:1-17.
- Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. 2020;0(0):39-45. doi:10.1515/cclm-2020-0240
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. doi:10.1016/S0140-6736(20)30566-3
- Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020:1-13. doi:10.1056/nejmoa2002032
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;(March). doi:10.1016/j.tmaid.2020.101623
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020:1-10. doi:10.1001/jamainternmed.2020.0994
- Gao Y, Li T, Han M, et al. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID‐19. J Med Virol. 2020;(March). doi:10.1002/jmv.25770
- Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505(March):190-191. doi:10.1016/j.cca.2020.03.004
- Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;(March):1-4. doi:10.1002/ajh.25774
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. doi:10.1016/S0140-6736(20)30628-0
- Siddiqi H, Mehra M. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J Hear Lung Transplant. 2020;(March). doi:10.1016/j.healun.2020.03.012
- Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. 2020. doi:10.1161/CIRCRESAHA.120.317055
- Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50(1):3-13. doi:10.1016/j.resinv.2012.02.001
- Koyama K, Katayama S, Tonai K, Shima J, Koinuma T, Nunomiya S. Biomarker profiles of coagulopathy and alveolar epithelial injury in acute respiratory distress syndrome with idiopathic/immune-related disease or common direct risk factors. Crit Care. 2019;23(1):283. doi:10.1186/s13054-019-2559-6
- Kondo T, Hattori N, Ishikawa N, et al. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res. 2011;12(1):32. doi:10.1186/1465-9921-12-32
- Nathani N, Perkins G, Tunnicliffe W, Murphy N, Manji M, Thickett D. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care. 2008;12(1). doi:10.1186/cc6785