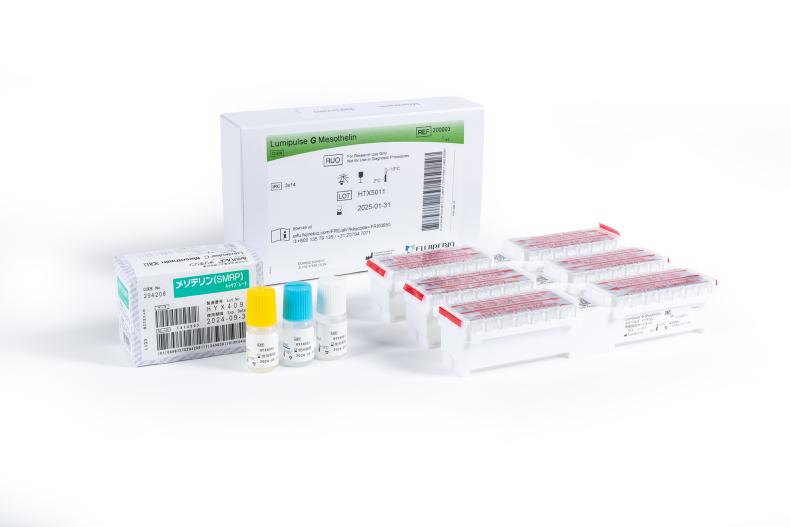
Lumipulse® G Mesothelin
For research use with the automated LUMIPULSE G System for the quantitative measurement of Soluble Mesothelin Related Peptides (SMRP) in human serum or plasma (dipotassium EDTA, sodium heparin, lithium heparin and sodium citrate).
This product is not for use in diagnostic procedures, for ‘Research Use Only’. This product is for professional use only.
For research use only
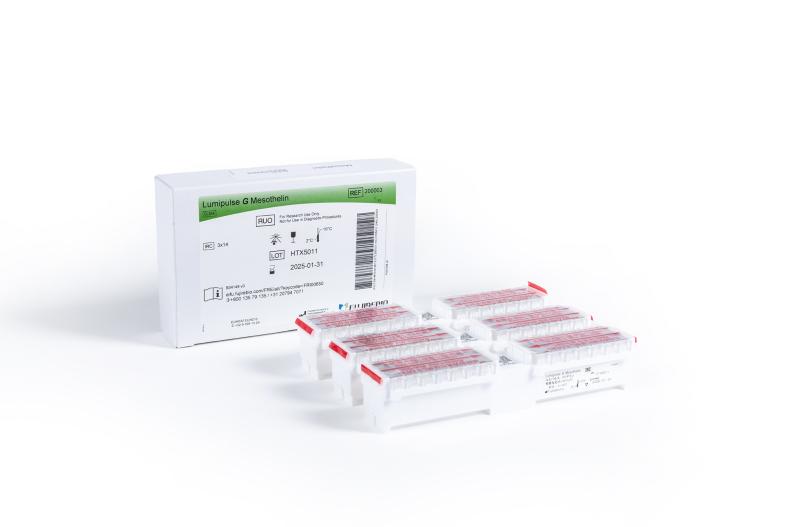
Lumipulse® G Mesothelin Immunoreaction Cartridges
Product number 200003
3 x 14 Tests
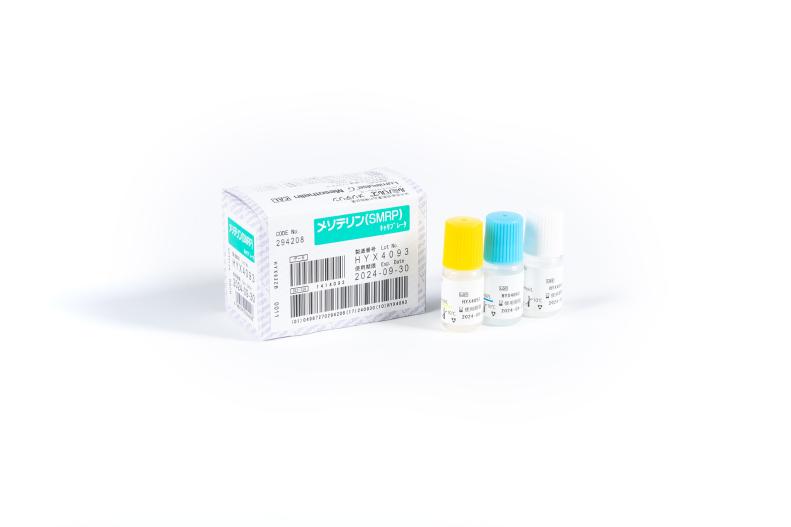
Lumipulse® G Mesothelin Calibrators
Product number 294208
1 x 3 Concentrations
Lumipulse® G Specimen diluent 1
Product number 231203
4 x 300 mL
Please contact your local Fujirebio representative for the availability of this product in your country.