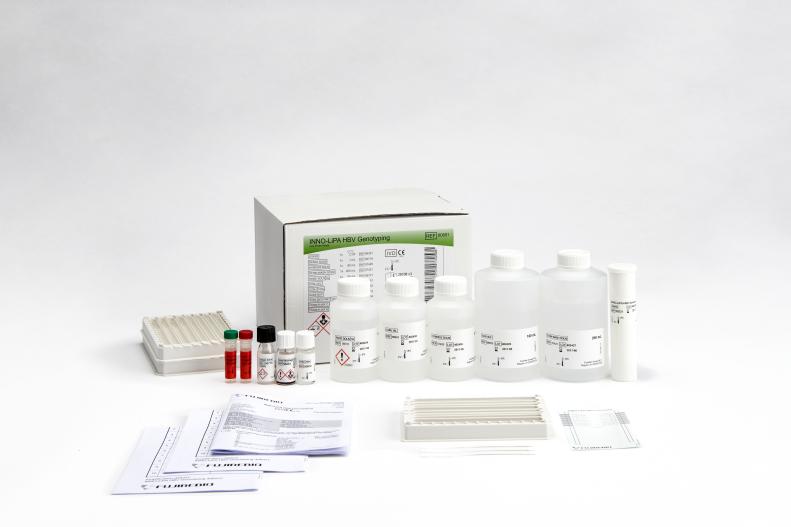
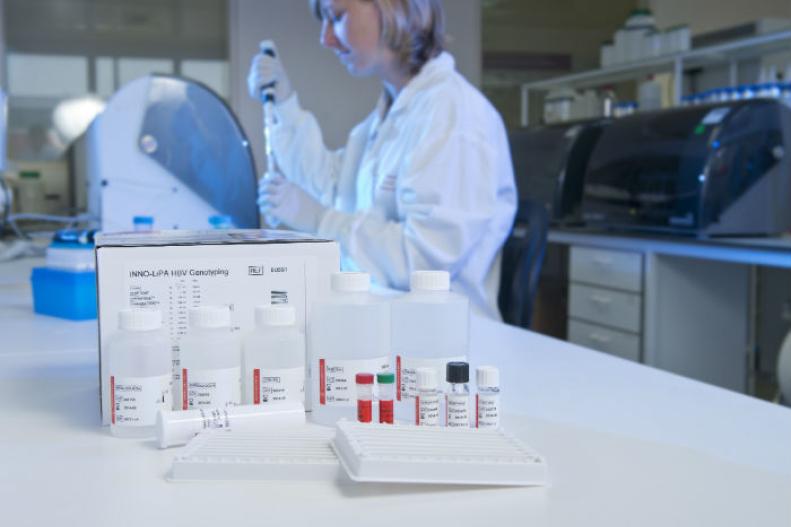
INNO-LiPA® HBV Genotyping
When genotyping, be confident
INNO-LiPA HBV Genotyping is a line probe assay designed to identify hepatitis B virus genotypes A to H by the detection of type-specific sequences in the HBV polymerase gene domain B to C.
Product number 80691
Click here to navigate
- Details
- Conditions of sale
- Citations
- Documentation
- Insights
- Related products
-
Details
Features & Benefits
- Most accurate results:
- Better detection of mixed genotype infections
- More sensitive than sequencing
- Confident choice of treatment
- Low chance of errors and limited hands-on time
- Easy to perform and robust assay
- Amplicon can also be used for INNO-LiPA HBV GT
- Fully automated processing of the strips possible with TENDIGO®, Auto-LiPA™ 48 and AutoBlot 3000H.
- Objective and automated reading and interpretation of strips possible using LiRAS® for LiPA HBV.
- One technology for a complete HBV portfolio.
- Most accurate results:
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Citations
The BIOZ badges associated with Fujirebio products include peer-reviewed citations derived from scientific studies using Fujirebio products. Please note that the peer-reviewed citations do not reflect the regulatory status of Fujirebio products. Users should refer to the specific product documentation and any (clinical) claims made therein in order to ensure compliant use. For each country or geographic region, users must verify the related regulatory status of the Fujirebio product.
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
Multimedia
Watch product videos
Get a (free!) eServices account and benefit from full access to all our online resources.
-
Insights
Jul 10, 2019HBcrAg, a promising new serological marker for reliable and non-invasive monitoring of the intrahepatic virologic status in HBV patients
In March 2017 the European Association for the Study of the Liver (EASL) added a new serological marker, HBcrAg (Hepatitis B core-related antigen), to...
-
Related products