Lumipulse® G β-Amyloid Ratio (1-42/1-40) CSF
The Lumipulse G β-Amyloid Ratio (1-42/1-40) is an in vitro cerebral spinal fluid (CSF) test that combines the results of Lumipulse G β-Amyloid 1-42 and Lumipulse G β-Amyloid 1-40 into a ratio of ß-Amyloid 1-42 to ß-Amyloid 1-40 concentration using the LUMIPULSE G1200.
For in vitro diagnostic use
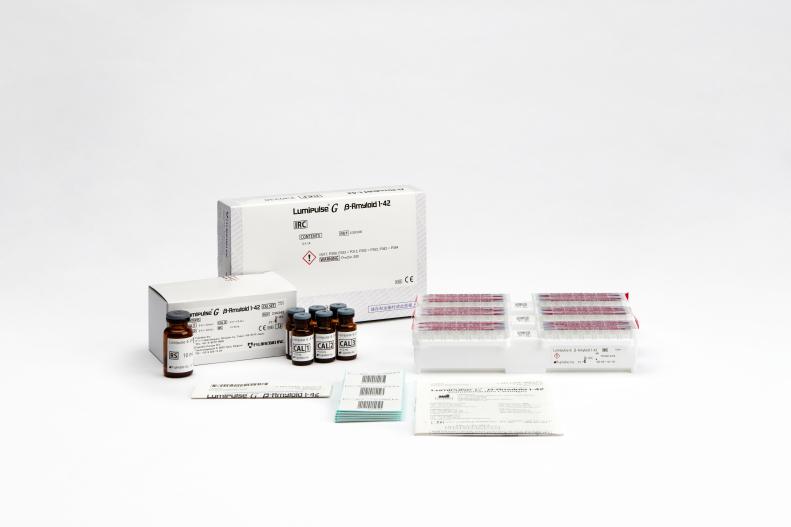
Lumipulse® G β-Amyloid 1-42 IRC
Product # 231432
3 x 14 Cartridges
Lumipulse® G β-Amyloid 1-42 Calibrators
Product # 234884
2 x 3 Concentrations
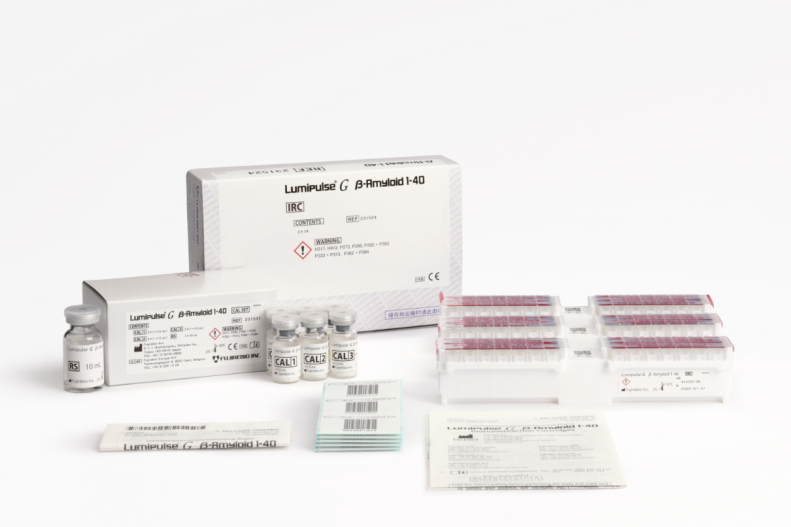
Lumipulse® G β-Amyloid 1-40 IRC
Product # 231753
3 x 14 Cartridges
Lumipulse® G β-Amyloid 1-40 Calibrators
Product # 234891
2 x 3 Concentrations
Lumipulse® β-Amyloid Controls
Product # 234907
2 x 3 Concentrations
Please contact your local Fujirebio representative for the availability of this product in your country.